da Harkaran Singh mancano 4 anni
240
ENERGY
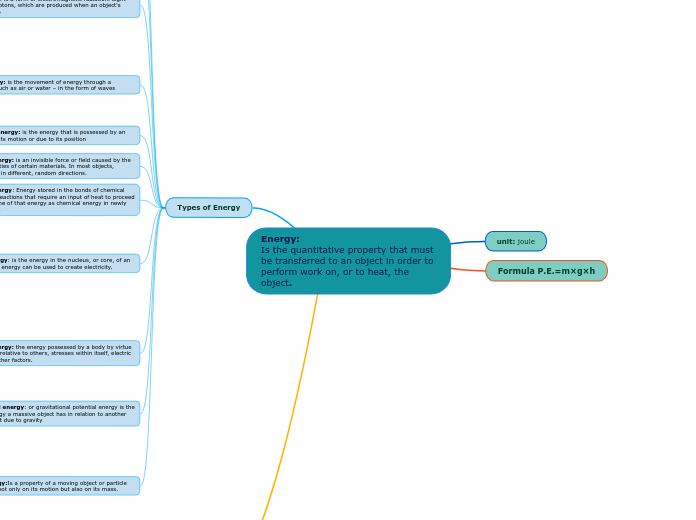
Energy is a fundamental concept involving the transfer required to perform work or heat an object. The conservation law dictates that energy cannot be created or destroyed, only transformed between forms.
da Harkaran Singh mancano 4 anni
240

Più simili a questo