によって ROMINA PIÑEIRO 5年前.
339
elements and compounds
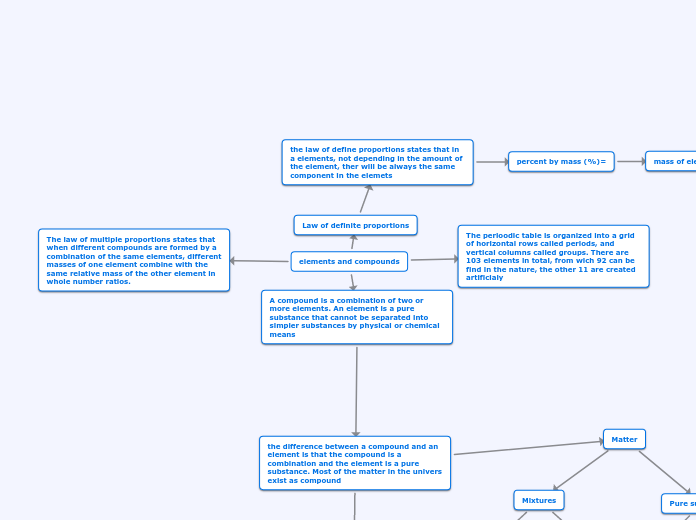
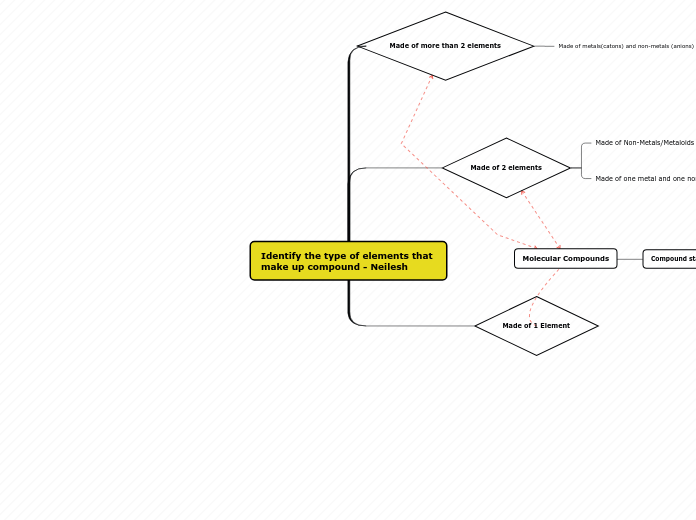
Elements are pure substances that cannot be separated into simpler substances by physical or chemical means, while compounds are combinations of two or more elements. Most matter in the universe exists as compounds, and separating them often requires external energy such as heat or electricity.