The Joining of Amino Acids and tRNA
1. The enzyme Aminoacyl-tRNA synthetases fits a unique combination of Amino Acid and tRNA. It begins by pairing the correct anticodon of the tRNA to the codon of the enzyme. The correct amino acid is also linked to the enzyme.
2. Hydrolysis of ATP occurs so the amino acid and tRNA form a covalent bond. Once bonded this is referred to as charged tRNA.
3. The Aminoacyl tRNA synthase releases the charged tRNA to be used in translation.
double helix
antiparallel strands
leading strands only only require one primer while the lagging strand requires multiple primers creating okazaki fragments (DNA polymarase III)
DNA polymarase I removes the RNA primers and replaces with DNA nucleotides
DNA ligase seals the gaps by connecting nucleotides by phosphodiester linkages.
Termination
1. A protein shaped like a tRNA, known as the release factor, is accepted into A site when the stop codon of the mRNA is reached.
2. This protein causes hydrolysis between the last amino acid and the tRNA in P site and the two separate, releasing the polypeptide from the ribosome.
3. The ribosome falls apart. All components of the ribosome separate from one another through the hydrolysis of two GTP.
Elongation
1. Codon Recognition - A charged tRNA
pairs it's anticodon with the mRNA codon
currently in the A site of the ribosome. Hydrolysis of GTP makes this process more accurate.
2. Peptide Bond Formation - The rRNA in
the large ribosomal subunit forms a peptide bond between the carboxyl end of the peptide chain and the new amino acid in A site. In doing this the amino acid and tRNA on P site disconnect and the tRNA is now empty.
3. Translocation - The empty tRNA moves to the E site and is released from the ribosome. The tRNA connected to the peptide chain is moved to the P site (the mRNA is shifted as well) and the A site is empty. The energy from GTP hydrolysis is used here. The cycle is then ready to be repeated.
Initiation
1. The small ribosomal subunit binds to the 5' end of the mRNA made in Transcription once it enters the cytoplasm.
2. The initiator tRNA pairs with the start codon on the mRNA.
3. The large ribosomal subunit attaches itself and the translation initiation complex is formed (this is due to the hydrolysis of GTP). When attached the tRNA is placed in the P site where the peptide chain can begin at the N-terminus with the amino acid MET.
Messleson and Stahl's experiment proved that the correct model of DNA replication is the semiconservative model
The ribosomal subunits are both made of rRNA. The small ribosomal subunit consists of an mRNA binding site. The large ribosomal subunit consists of three tRNA binding sites, consisting of A, P, and E, which the tRNA goes through in that order.
Protein is then shipped to the Golgi through a vesicle
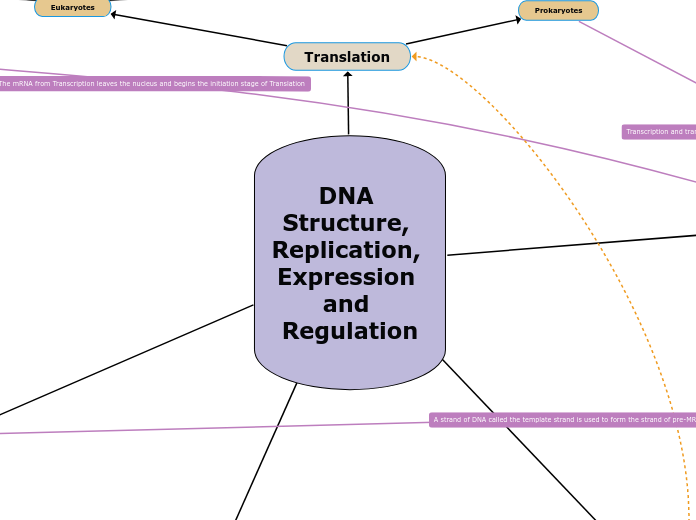
DNA Structure, Replication, Expression and Regulation
Gene Regulation
Gene regulation can occur at any step in gene expression including transcription, mRNA processing, translation, and protein transport.
Transcription is the most critical step for regulating gene expression because gene regulation can block transcription of a certain gene.
Gene Regulation at Transciption
Control elements in DNA bind transcription factors
Types of Control Elements
Distal Control Elements
Sequences in DNA called enhancer sequences that bind specific transcription factors (activators and repressors). Enhancer sequences may be upstream or downstream of a gene and close to or far from the gene they control.
A DNA-bending protein brings the bound activators/repressors closer to the promotor, and RNA polymerase II binds to the promoter, initiating trancription.
Proximal Control Elements
Sequences in DNA close to the promoter that bind general transcription factors
Transcription factors are proteins that bind at the promoter sequence on the DNA strand, increases the binding affinity for the RNA polymerase to recognize the promotor sequence and bind to it, and initiate transcription.
Types of Transcription Factors
Specific
Changes level of transcription
Types of Specific Transcription Factors
Repressors
Reduce levels of transcription down to background level
Activators
Increase levels of transcription above background level
General
Bring about low levels of transcription (background/basal)
Cell specific transcription: combinatorial control of gene expression to increase or decrease expression of different genes
All cells in the body have the same DNA and same genes. Differences in function between cell types result from differential gene expression, the expression of different genes by cells with the same genome.
In prokaryotes, transcription and translation are coupled processes and occur in the cytoplasm because there is no nucleus.
Gene regulation occurs at the level of transcription.
Operons are a cluster of functionally related genes that can be under coordinated control of a single on-off “switch”. The "switch" is a segment of DNA called an operator which is usually positioned within the promoter.
The Lac operon uses both activators and repressors and is an example of both positive and negative regulation.
Glucose in bacterial cell
Adenyl cyclase active
cAMP levels high
CAP active
Adenyl cyclase inactive
cAMP levels low
CAP inactive
Lactose in bacterial cell
Not present
Repressor bound to operator
Lac operon is OFF
Present
Repressor bound to allolactose
Lac operon is ON
Types of Gene Regulation in Prokaryotes
Negative Regulation
Repressor proteins bind operator sequences to decrease expression down to basal level or stop transcription of a gene
Operon is OFF
Positive Regulation
Activator proteins bind operator sequences to increase expression above the basal level
Operon is ON
DNA
REPLICATION
process
primase makes RNA primers complementary to the DNA parent strand sequence
then, DNA polymerase III will add nucleotides only to the 3' end
replication bubble is made at the Origin of Replication (ORI)
Topoisomerase relieve the tension caused by the unwinding of the DNA
Single-Stranded proteins keep the DNA from coming back together
helicase separates the two strands by breaking the hydrogen bonds between the strands
three proposed models of DNA replication
dispersive model
each strand contains parts of both old and newly synthesized DNA
semi-conservative model
the parental strands separate and makes its own new complimentary strand
conservative model
the two parental strands are used as templates, they stay together
STRUCTURE
nitrogenous bases (hydrogen bonding) make up nucleotides
guanine
cytosine
thymine
Adenine
sugar-phosphate backbone
connected by phosphodiester bonds
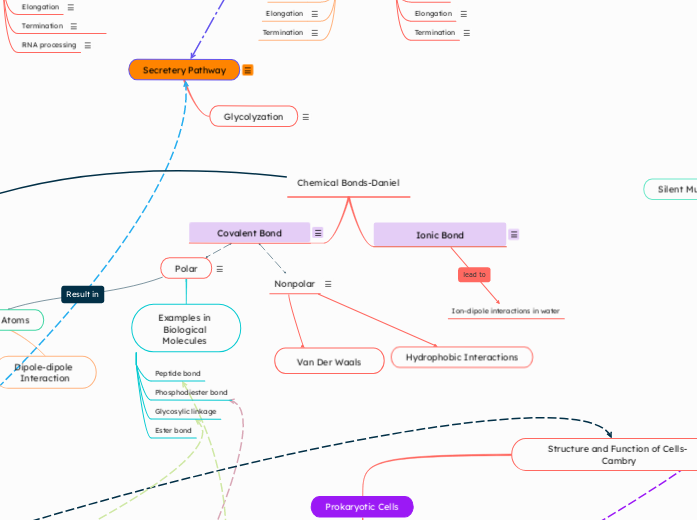
Translation
Takes place immediately after Transcription since both take place in the cytoplasm here. The mRNA is still translated into polypeptides here through the same steps.
Transcription
3 STAGES
Termination: RNA transcript is released. Polymerase detaches from the DNA
Elongation: New RNA nucleotides are added to the 3' end
Initiation: Start of transcription at +1
Prokaryotes
Location: Cytoplasm
Forms: mRNA
Initiation Enzyme: RNA Polymerase
Eukaryotes
Location: Nucleus
Forms: pre-mRNA then mRNA thru RNA Processing
Initiation Enzyme: RNA Polymerase II
Needs TRANSCRIPTION FACTORS that bind near the promoter before RNA Poly II can bind. TF recognize the TATA box in promoter sequence.
RNA Poly II binds to the promoter upstream the start site
TF + RNA Poly come together to form the translation initiation complex
Termination: 5' cap and 3' Poly A tail
Termination Enzymes:
Ribonuclease: Cleavage
PolyA polymerase: adds poly A tail, Uses ATP
RNA Processing: Removing of introns and the joining of exons
Spliceosomes: cut out the introns
Protein Transport
Takes secretory pathways. The path taken by protein in a cell on synthesis to modification and then release out of the cell.
Destination of synthesis completed on free ribosomes
All protein synthesis starts on free ribosomes.
Endomembrane System
Polypeptide synthesis begins on free ribosome in cytosol
Synthesis stops temporarily from SRP binding to signal peptide
SRP is signal recognition particle that is made of RNA and protein.
SRP binds to receptor protein in ER membrane
SRP leaves, polypeptide synthesis resumes
Signal peptide is removed by an enzyme in receptor protein complex
Signal peptidase is the enzyme used to cleave the signal peptide.
Protein synthesis finishes inside the ER
Cytoplasm
Organelles
Nucleus
Peroxisomes
Chloroplast
Mitochondria