by Sam Bartlett 6 years ago
345
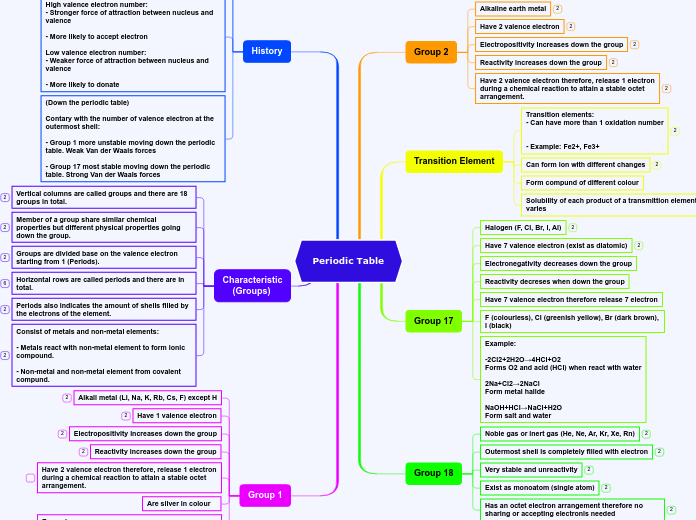
Structure and Bonding
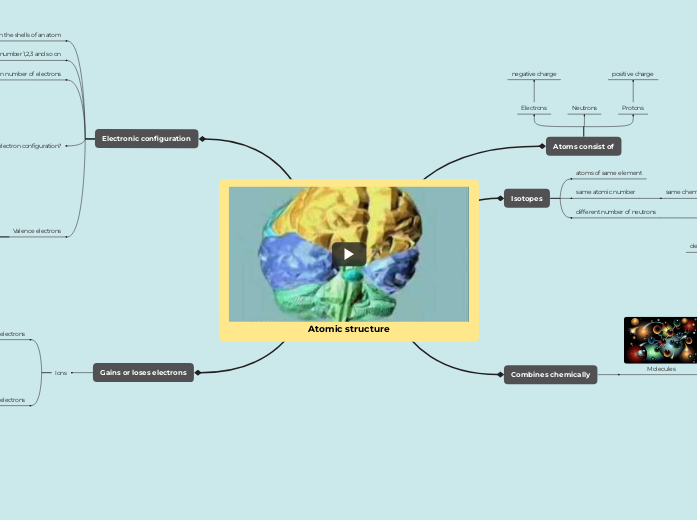
Ions are particles created when atoms lose or gain electrons to achieve full outer shells, typically occurring during reactions between metals and non-metals. Metal atoms lose electrons to form positive ions, while non-metal atoms gain electrons to form negative ions.