by Angelica Rodriguez 6 years ago
320
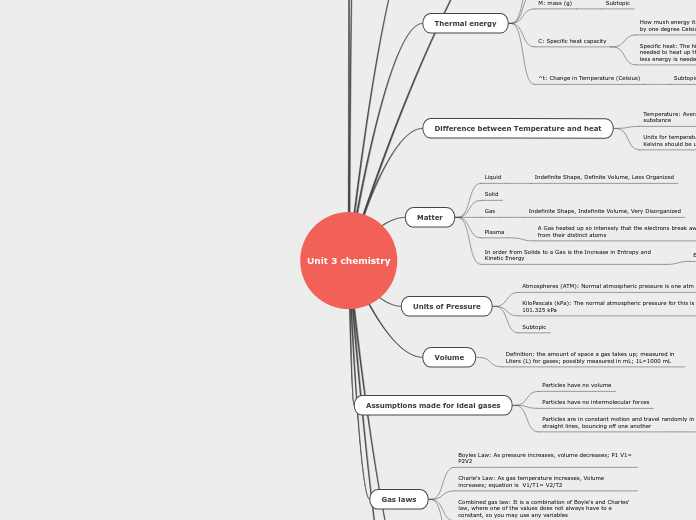
Unit 3 Chemistry
The study of thermal energy in chemistry involves understanding how heat is measured in joules or calories and the specific heat capacity of substances. Specific heat indicates the amount of energy required to change the temperature of one gram of a substance by one degree Celsius.