by Aina Nazurah 4 years ago
398
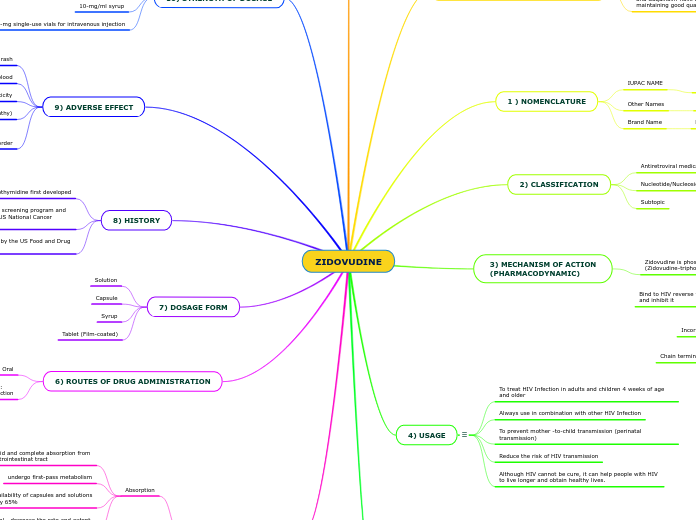
ZIDOVUDINE
Zidovudine, initially developed in 1964, became notable for its approval by the US Food and Drug Administration in March 1987. The drug, primarily used to treat HIV infections, operates by incorporating into viral DNA and causing chain termination, thereby inhibiting the replication of the virus.