The further it gets from neutral the stronger acid or base is
Difference in electronegativity
more than 1.7
more than zero bigger or equal to 1.7
equals to zero
Non-polar molecule
If there are any lone pairs (it means nonbonding) on the central atom, then the molecule is polar. but there are exceptions: linear and square planer
If all electron density regions are the same which they should be all bonded electrons then the molecule is non-polar because of the symmetry
PH scale
Neutral(7)
Between 7.1-14
Between 0-6.9
First ionization energy
How easy it is for an atom to lose electron. Easier to lose= lower ionization energy
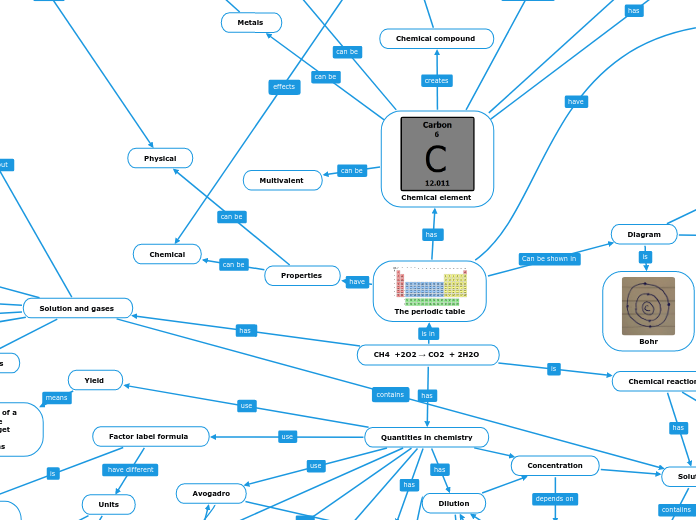
CH4 +2O2 → CO2 + 2H2O
Chemical reaction
Reaction/No reaction
Chemical equation
Balancing
Law of conservation of mass (Lavoisier)
In chemical reactions, no mass is lost or gained.
Solution
Solvent
The pure substance that will dissolve the solute.
Solute
The pure substance being dissolved
Solubility
The measurement of how well a solute will dissolve in a solvent at a given pressure and temperature
ability of a solute to dissolve
Insoluble
Percipitate
a solid formed in a chemical reaction
Soluble
word equation
Methane reacts with Oxygen gas to produce Carbon dioxide and water.
Types
Displacement
Single
When a free element replaces with a less active element that is in a chemical compound. (based on reactivity charts of metals and non-metals)
Double
Double displacement will not happen unless either water or a precipitate is being formed.
To predict if the double displacement reaction can occur or not we should use solubility rules chart.
Solubility rules
Neutralization
This type of reaction happens when an acid and a base combine to form water and salt.
Synthesis
When two or more reactants combine and form a new product
Combustion
When a fuel (especially hydrocarbons) burns with oxygen to form carbon dioxide and water.
Decomposition
When one reactant breaks to form multiple products
Quantities in chemistry
Yield
How much of a product we expect to get based on calculations
Percent Yield=Actual Yield/Theoretical Yield x 100%
Molar mass
Mass
limiting and excess reactants
In chemistry we use stoichiometry to determine which reactant yields the smaller mass of product in a chemical reaction.
Stoichiometry
The study of quantitative relationships in chemical reactions
Dilution
Final
Initial
Stock solution
Concentration
n
V
C
Avogadro
Avogadro discovered in exactly 12g of carbon there were 6.02 x 10*23 carbon atmos. this number is called ''Mole''
1 Mole=6.02 x 10*23 things
Factor label formula
What you can find=what you know X the fraction(s)you need to get to the answer
We use this formula to switch back and forth between different units and muasurments
Units
L
Kg
Solution and gases
Indicators
indicators are chemicals that when it is dissolved in acid or bases it would change the colour so it would make it easier for us to recognize them.
Most acid and bases are colourless and clear it's hard to distinguish them so it's better to use indicators
Bronsted-Lowry Acid and Bases
When an acid and base are mixed together, the acid will transfer a proton to the base.
HCL + H2O = H3O + CL
Chlorine gas ( The conjugate base)
Hydronium (The conjugate Acid)
Water (The Bronsted Lowry base)
hydrochloric acid (The Bronsted Lowry acid)
Bases
Potassium hydroxide(KOH)
Most of the bases contain a metal and a hydroxide polyatomic group.
Substances that produce hydroxide ions and take a proton from another compound.
Acids
Substances that produce hydrogen ions in solution and donate proton to another compund.
Oxyacids
It contains hydrogen and a polyatomic that has hydrogen.
''ic'' acids
Nitric acid(HN3)
''Ous''acids
Nitrous acid(HNO2)
Binary
contains a hydrogen and another nonmetal
hydrochloric acid (HCl)
Solubility curves
The relationship between solubility and temperature can be expressed by a solubility curve.
Solubility of CH4 in water
Solubility curves shows the maximum amount of solute that can be dissolved in a given amount of water over a range of temperatures.
Reading solubility curves
Super Saturated
Above the line
Saturated
Directly at the line
Unsaturated
Below the line
Titration
Is the process to measure the concentration or volume of a substance by adding a certain amount of another substance
The periodic table
Trends
Electronegativity
Tendency of an atom to attract shared pair of electrons within a molecular bond.
Electronegativity of elements
Affinity
How easy it is for an atom to gain electron. Easier to gain= higher electron affinity
Reactivity of non-Metals
How easily a non-metal would become an anion.
Reactivity of Metals
How easily a metal would become a cation.
Atomic radius
The distance from nucleus to the outermost shell.
Properties
Chemical
Physical
Diagram
Bohr
Orbital
Lewis
compound lewis structure
Chemical element
Metals
Cation
Non-metals
Anion
Isotopes
Average weight
Multivalent
Atomic mass
atoms
Atomic theory
Jemes Chadwick model
Bohr model
Rutherford and Nuclear model
Thomson model
Dalton model
Greek model
Electron
Neutron
Proton
Chemical compound
bond polarity(Intramolecular forces)
This type of force is within the molecules(strong)
The type of bond will be determined by the difference in electronegativity of two atoms.
Ionic
Transfer of electrons
Molecular
sharing electrons
Intermolecular forces(molecule polarity)
This type of forces is between the molecules(weak)
Hydrogen bonding
A special type of dipole-dipole which occurs between hydrogen and either oxygen, nitrogen and fluorine. This intermolecular has a strong attraction because of big difference in electronegativities.
Dipole-Dipole
This intermolecular force forms between a slightly negative end of one Polar molecule and a slightly positive end of a neighbouring molecule causing them to pull towards each other
London dispersion
Electrons are always moving, sometimes they bunch up on one side of an atom, causing them to be slightly negative.
If this happens to a neighbouring molecule at the same time there would be a temporary attraction called London dispersion.
Polarity
Non-polar
Polar
Vsper shape
Vsepr shape allows us to predict the individual molecule structure and it's polarity based on the number of electron pairs that surround the center atom.
Valence shell electron pair repulsion theory