Equilibrium
Main topic
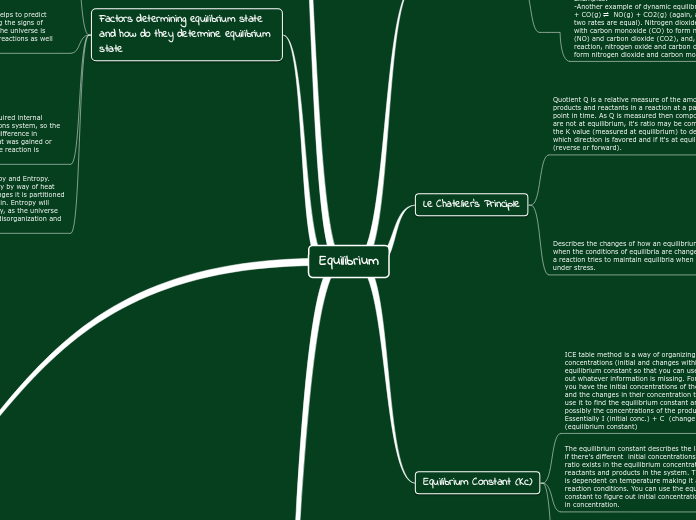
Factors determining equilibrium state and how do they determine equilibrium state
The compromise between Enthalpy and Entropy. Enthalpy is the measure of energy by way of heat loss or gain as the energy is changes it is partitioned and this is where entropy comes in. Entropy will make it more towards spontaneity, as the universe always heads in the direction of disorganization and chaos.
Enthalpy (∆H) describes the required internal energy or pressure for the reactions system, so the enthalpy change is equal to the difference in constant pressure or the heat that was gained or lost, essentially determining if the reaction is exothermic or endothermic.
The gibbs free energy equation helps to predict reaction spontaneity by observing the signs of enthalpy and entropy terms. As the universe is always becoming more random, reactions as well favour the
If factors are balanced, forward and reverse ca n be equally favoured/ spontaneous.
∆H | ∆S | ∆G |Which direction is spontaneous?
| | 0 |
Forward more favoured/ spontaneous at high temp.
∆H | ∆S | ∆G |Which direction is spontaneous?
+ | + | - if T is large |
Forward more favoured/ spontaneos us at low temp.
∆H | ∆S | ∆G |Which direction is spontaneous?
- | - | - if T is small |
Forward does not occur. The reverse is favoured/ spontaneous at any temperature.
∆H | ∆S | ∆G |Which direction is spontaneous?
+ | - | Always + |
Forward is favoured/ spontaneous at any temperature. The reverse does not occur.
∆H | ∆S | ∆G |Which direction is spontaneous?
- | + | Always - |
Entropy ( ∆S) is a measurement of chaos, randomness or disorder of a system. In the universe entropy is always expanding. Enthalpy may be augmented if there is a greater accumulation of particles present in a system or if the particles are made into more unstable/disorganized states such as gases.
Second Law of thermodynamics states that in equilibrium entropy stays the same and it will otherwise increase until equilibrium is reached. This leads to Boltzmann's theory of entropic increase with molecular energy, he suggested that if the final state in a system is random then the initial must be the opposite. (∆S system + ∆S surroundings always equals a +∆S universe ).
Entropic Changes :
Phase Changes such as vaporization and fusion may suggest and increase in entropy
Chemical equilibrium
Example :
- The release of CO2 when you open up a soda (carbonated drink)
CO2(g)+H2O(l)⇌H2CO3(aq)
The carbon dioxide is constantly moving from liquid to gas phase but due to the equal rates in which the forward and reverse reactions occur, no change can be observed.
Reversible reaction
A state in a chemical system where the forward and reverse reactions occur simultaneously and at the same rate (only in chemical reactions). The concentrations of the reactants and products do not change.
Equilibrium Constant (Kc)
The equilibrium constant describes the law that even if there's different initial concentrations, a constant ratio exists in the equilibrium concentrations of reactants and products in the system. This constant is dependent on temperature making it attached to reaction conditions. You can use the equilibrium constant to figure out initial concentrations, changes in concentration.
ICE table method is a way of organizing the concentrations (initial and changes within it) and equilibrium constant so that you can use it to figure out whatever information is missing. For example if you have the initial concentrations of the products and the changes in their concentration then you may use it to find the equilibrium constant and then possibly the concentrations of the products. Essentially I (initial conc.) + C (change in conc. )= E (equilibrium constant)
Subtopic
Le Chatelier's Principle
Describes the changes of how an equilibrium shifts when the conditions of equilibria are changed; when a reaction tries to maintain equilibria when being put under stress.
An example of Le Chateliers principle can be seen in the purposeful colour changes of Hydrangeas. Gardeners will change the acidity of the soil so that the equilibria of the aluminum solubility will be thrown off so it will absorb more aluminum and turn blue.
Examples or stressors on a chemical system includes the addition or removal of a product (common ion effect) and the changes in pressure, temperature and addition of a catalyst, (messing up the rates of the reaction). The stressors may speed up the reaction (commom ion eftect by increasing amount of chemicals involved) or change the relative spontaneity of forward and reverse reactions (change in temp) and pressure changes will result in a favouing of the side with less gas.
Quotient Q is a relative measure of the amount of products and reactants in a reaction at a particular point in time. As Q is measured then components are not at equilibrium, it's ratio may be compared to the K value (measured at equilibrium) to determine which direction is favored and if it's at equilibrium (reverse or forward).
< K The system has excess reactants. The forward reaction will go faster to reach equilibrium.
If Q is = K the system is already at equilibrium. Forward and reverse reactions are at the same speed
if Q is... | Then...
> K | the system has excess products. The reverse reaction will go faster to reach equilibrium
Dynamic Equilibrium
A chemical system at equilibrium with static macroscopic properties but transitions on a molecular level (bonding, breaking of bonds), the rate of the forward reaction equals the rate of the reverse reaction as well.
Reversible in nature
Can only occur in closed systems
Macroscopic properties are categorized as measurable traits (some qualitative some quantitative) such as color, pH, size, concentration