Gibbs energy
Gibbs free energy relates entropy and enthalpy and they can be used to predict if a reaction is spontaneous.
Formula: ΔG = ΔH-TΔS
Negative ΔG represents the amount of wok that can be extracted from the process or reaction.
If ΔG>0, then there will be a NON-spontaneous reaction
If ΔG<0, then there will be a spontaneous reaction
If ΔH is higher than ΔS, ΔG will be more positive.
If ΔH is lower than ΔS, ΔG will be more negative.
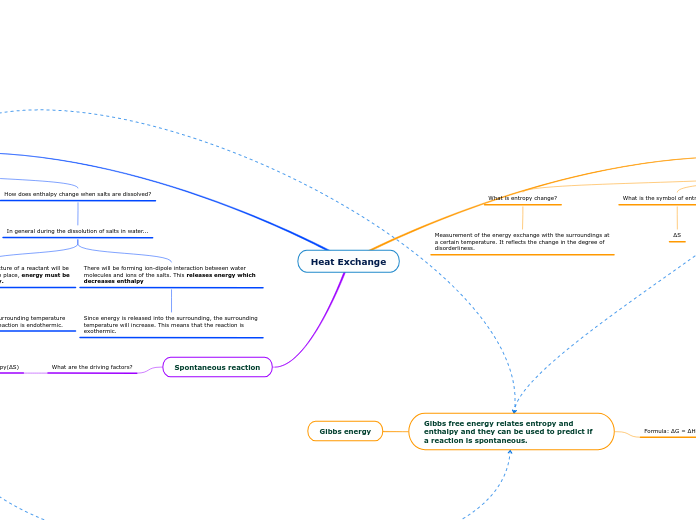
Heat Exchange
Spontaneous reaction
What are the driving factors?
Ethalpy(ΔH) and Entropy(ΔS)
Enthalpy change
How does enthalpy change when salts are dissolved?
There will be forming ion-dipole interaction between water molecules and ions of the salts. This releases energy which decreases enthalpy
Since energy is released into the surrounding, the surrounding temperature will increase. This means that the reaction is exothermic.
Ionic bonds in the crystal lattice structure of a reactant will be broken down. In order for this to take place, energy must be taken in which increases enthalpy.
Since energy must be taken in, the surrounding temperature will decreases. This means that the reaction is endothermic.
What is the symbol of enthalpy change?
ΔH
What is enthalpy change?
Measurement of the heat exchange with the surroundings under constant pressure. It reflects the breaks and forming of inter- or intra-molecular interactions.
Entropy change
How does entropy change when salts are dissolved?
In general during the dissolution of salts in water...
When there is change of state between the reactant and the products, there will be a change in entropy.
Entropy decreases when the particles gets more organised or ordered.
Example: H20(l) -> H20(s)
Entropy increases when the particles get more disorganized or disordered.
Example: NaCl(S) + H20(l) -> NaOH(aq) + HCl(aq)
There is a change of state between the reactant, NaCl, and the product, NaOH. This causes entropy to increase because the there is a greater disorderliness in the product than the reactant.
What is the symbol of entropy change?
ΔS
What is entropy change?
Measurement of the energy exchange with the surroundings at a certain temperature. It reflects the change in the degree of disorderliness.