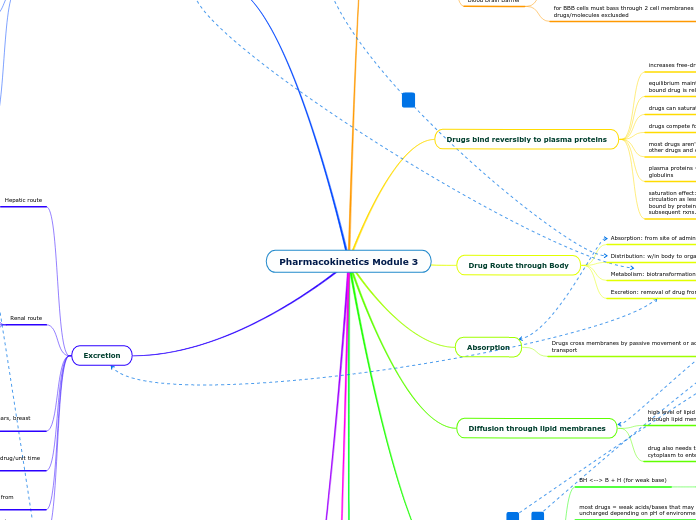
Pharmacokinetics Module 3
ATP-binding cassette (ABC) Transporters
P-glycoproteins (P-gp)
responsible for drug resistance in cancer (mediate removal of drugs from cells)
ATP-binding transporters
transport against concentration gradients
Organic Cation/Anion Transporters
Solute Carriers (SLC)
transports single species in direction of its electrochemical gradient
facilitated diffusion
structurally related to organic cation/anion transporter (OCT and OAT)
Passive movement of solutes down gradients
mediate cell uptake/movement across membrans
Excretion
total body clearance: sum of all clearances by various mechanisms
Drug elimination: measure of loss of drug mass from circulation/unit time (mg drug/hour)
includes loss of drug by metabolism and excretory routes
Plasma Clearance: volume of plasma cleared of drug/unit time (ml/min)
measure of efficiency of organs of elimination (wellness of patient)
reduced clearance = impairment of excretory organ
clearance is constant for a drug since volume of distribution, bioavailability and drug half life are constant
also excreted via feces, breath, sweat, saliva, tears, breast milk
Renal route
pH of urine can vary due to diet and drug intake
99% water filtered is reabsorbed long tubule, thus drugs are reabsorbed due to concurrent resorption of water
lipophilic drugs cross membranes easier and are reabsorbed more than polar hydrophilic molecules
ion trapping increases drug retention in the urine
weak acids excreted more in alkaline urine
weak acids excreted less in acidic urine
passive diffusion across tubular epithelium
lipophilic drugs and those non-ionized in urine can be reabsorbed
active tubular secretion
involves specific transport carriers (OAT, OCT)
can be antagonized
glomerular filtration
plasma proteins (albumin) don't pass bc too large
**warfarin is 98% bound to albumin (2% avail for filtration)
plasma protein bound drugs aren't filtered
molecules smaller than 5000-15000 can pass
Hepatic route
Enterohepatic circulation can re-uptake excreted drugs
losing conjugate through hydrolysis = reactivation of drug and reabsorption by body
results in prolonged drug action
drugs/substances transported from plasma to bile via SLC and P-gp transporters
Metabolism
Liver = main site for drug metabolism
Phase 2 Metabolism: conjugation of side chains to drugs
normally result in inactive product
can conjugate to residue created by phase 1 rxns
mainly take place in liver; but also lung/kidney
through glucuronidation, sulphation, glutathione addition, methylation, glycine addition, and water conjugation
produces polar molecule that can be excreted
Phase 1 Metabolism: oxidation runs, reduction and hydrolysis
not all drug oxidation is cytochrome liver system
INSTEAD: can occur in plasma or other tissues
mediated by cytochrome system (P450NZ)
genetic variations in p450 NZs = variation in response to drugs
p450 can be regulated by external factors (grapefruit juice, smoke)
iron bearing haem proteins (CYP1/2/3)
cytochromes (hepatic cytoplasmic microsomal drug metabolizing NZs) are in the liver
oxidation adds O = hydroxylation, oxidation, dealkylation, deamination - increase water solubility
adds reactive residue to processed molecule
Subtopic
first pass effect = metabolize drugs before reaching circulation
in gastric mucosa or after absorption from gut into hepatic portal vein (in liver)
can also produce metabolites more active AND more toxic than parent drug
metabolic activity also makes metabolites more active than original molecules (pro-drugs)
reduces bioavailability of drug before reaching systemic circulation
alters structure to reduce its intrinsic efficacy
makes it more water soluble/less re-absorbed by renal tubule
pH partition and ion trapping
ion trapping: drug in compartment favoring its ionization/dissolution
BUT diffusion at eq. means fractions of non-ionized drugs can be found in compartments of varying pH
basic drug accumulates in compartment with low pH
acidic drug accumulates in compartment with high pH
pH favoring ionization = accumulation in compartment
pH favoring non-ionized form = more likely to pass from aq compartment
weak acid (pKa 3.9) in more basic environment (pH 8) is ionized
pH-pKa >0
readily dissolves
weak acid (pKa 3.9) in acidic environment (pH 2) is nonionized
pH=pKa < 0
greater lipid solubility
pH and ionization
protonation of a base charges that molecule
more likely to be ionized/not absorbed [BH+]
Protonation of an acid make the molecule neutral
more likely to be absorbed [HA]
knowing pKa of drug and pH of body fluid --> calculate proportion of drug ionized/how ready it crosses lipid membranes
if pH is high relative to pKa of weak acid drug its more likely to be changed and less lipid soluble
as pH increases relative to pKa of basic drug = nonionized and is lipid soluble
pH-pKa = log[A]/[HA]
pH - pKa < 0
non-ionized
pH - pKa > 0
ionized
THESE describe ionization state of drug at equilibrium
pH-pKa = log[B]/[BH]
most drugs = weak acids/bases that may be ionized or uncharged depending on pH of environment
uncharged forms pass through lipid membranes
ionized drugs dissolve well in aq fluids
BH <--> B + H (for weak base)
EQ1: Ka = [B][H]/[BH] (dissociation constant)
EQ2: -logKa = -log[H] - log [B]/[BH]
EQ3: pKa = pH + log[BH]/[B]
Diffusion through lipid membranes
drug also needs to be able o pass through aqueous cell cytoplasm to enter blood, intestinal secretions, and urine
equal levels of ionization in oil/water
high level of lipid solubility = drug better able to interact/pass through lipid membrane
too much lipid solubility can = drug staying in membrane (not passing)
partial co-efficient: ratio of drug dissolving in oil/water
Absorption
Drugs cross membranes by passive movement or active transport
Small water soluble molecules go through aqueous pores
Pinocytosis for large molecules
Specific carrier (majority of drugs)
SLCs/ABCs can transport drugs into and out of cells (influx and efflux)
Diffusion = nonspecific (majority of drugs)
Drug Route through Body
Excretion: removal of drug from body
Metabolism: biotransformation of drug; often inactivating
Distribution: w/in body to organs and tissues
Absorption: from site of administration
Drugs bind reversibly to plasma proteins
saturation effect: doubling dose increases free conc. of drug in circulation as less drug reaches circulation and is instead bound by proteins. The unbound free drug is available for subsequent rxns.
plasma proteins = albumin, lipoproteins, glycoproteins, beta globulins
most drugs aren't bound in sufficient quantities to displace other drugs and drastically alter free concentrations
drugs compete for binding to plasma proteins
depends on affinity
drugs can saturate binding sites on plasma proteins
equilibrium maintained as free drug goes into tissues and bound drug is released from proteins into free circulation
increases free-drug conc. in plasma
Distribution
Blood Brain Barrier
for BBB cells must bass through 2 cell membranes so drugs/molecules exclusded
AA, glucose, amines, purines are actively transported across BBB by carriers
lipophilic drugs cross
lacks fenestrations (small gaps that allow passage via diffusion)
Volume of Distribution: Vd = D/C0
take blood sample to see if drug is in tissues or in circulation
high Vd means drug has left circulation
low Vd means drug is primarily in plasma (still in circulation)
C0 = plasma conc. at time 0
D = dose
trans=cellular fluid (cerebrospinal, intraocular, peritoneal, pleural, synovial fluids, digestive secretions) = 2%
plasma water (5%), body fat (20%), interstitial water (16%) also are reservoirs
intracellular water = aqueous cytoplasm =cheif reservoir for water soluble drugs (35%)