door akia terry 12 jaren geleden
583
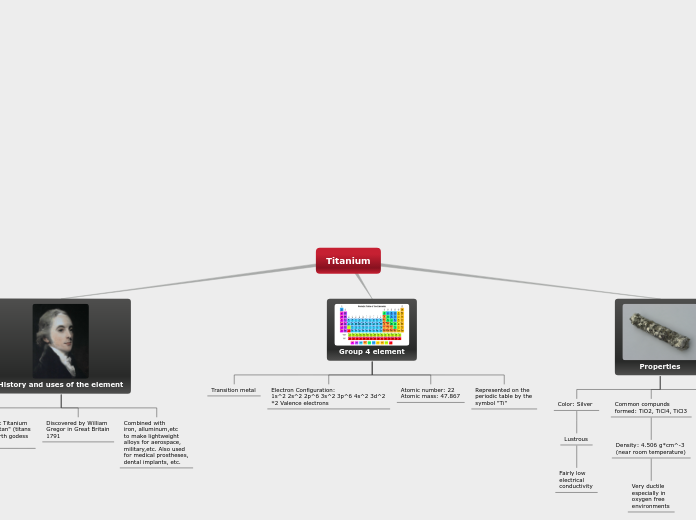
Titanium
The element titanium, named after the Greek mythological Titans by Martin Heinrich, holds significant importance in various fields due to its unique properties. Discovered by William Gregor in 1791 in Great Britain, titanium is celebrated for its lightweight and strength, making it ideal for aerospace and military applications when combined with other metals like iron and aluminum to form alloys.