av Boparai Harreet 2 år siden
228
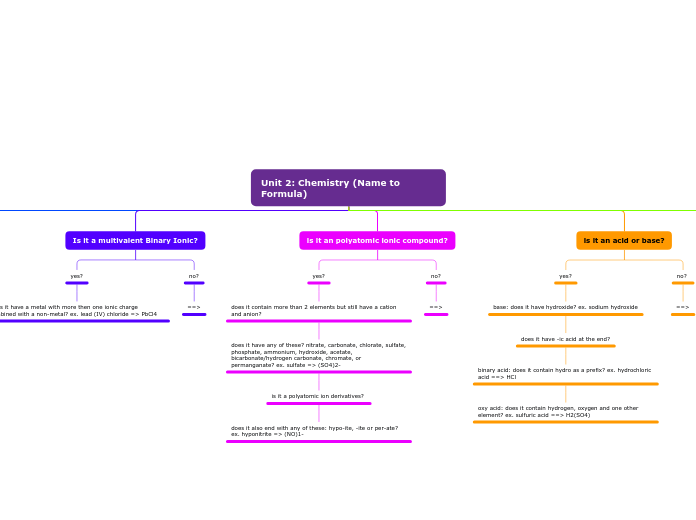
Unit 2: Chemistry (Name to Formula)
av Boparai Harreet 2 år siden
228

Mer som dette
Keeps the name and has a 2 next to it represnting that it has the 2 of the same atoms. ex. H2 = hydrogen
binary acid: hydro should be the prefix. then element name, which ends with -ic acid. ex. HBr => hydrobromic acid
oxy acid: polyatomic ending "ate" is removed and replaced with -ic acid. ex. H2CO3 => carbonic acid
is it a multivalent?
yes? Then the name of the metal goes first. Afterwards, add Roman numerals in brackets. the non-metal ends with -ide. ex. tin (II) oxide
is it a diatomic?
is it written as: (naturally have 2 of the same atoms) hydrogen - h2, nitrogen - N2, chlorine - Cl2, iodine - I2, oxygen - O2, fluorine - F2, bromine - Br2
does it have -ic acid at the end?
binary acid: does it contain hydro as a prefix? ex. hydrochloric acid ==> HCl
oxy acid: does it contain hydrogen, oxygen and one other element? ex. sulfuric acid ==> H2(SO4)
does it have any of these? nitrate, carbonate, chlorate, sulfate, phosphate, ammonium, hydroxide, acetate, bicarbonate/hydrogen carbonate, chromate, or permanganate? ex. sulfate => (SO4)2-
is it a polyatomic ion derivatives?
does it also end with any of these: hypo-ite, -ite or per-ate? ex. hyponitrite => (NO)1-