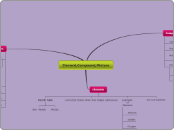
Element,Compound,Mixture
Compound
Cannnot be physically seperated but can be seperated by chemically methods
Propertion of Copound is different from the Elements
Made up of 2 or more different Elements chemically combined
Examples of Compound
Salt
Sugar
Elements
Perodic table
Non -Metals
Metals
cannot be broken down into simpler substances
Examples of Elements
Chlorine
Sodium
Oxygen
Hydrogen
Are pure subtances
Mixture
Examples of mixtures
Seawater
Gasoline
Made up of 2 or more different substances(Elements and Compound ) that are mixed together
Can be seperated by physically means
Are not fixed in propertion
They are not chemically combined