realizată de Kaffa Milatina 3 ani în urmă
472
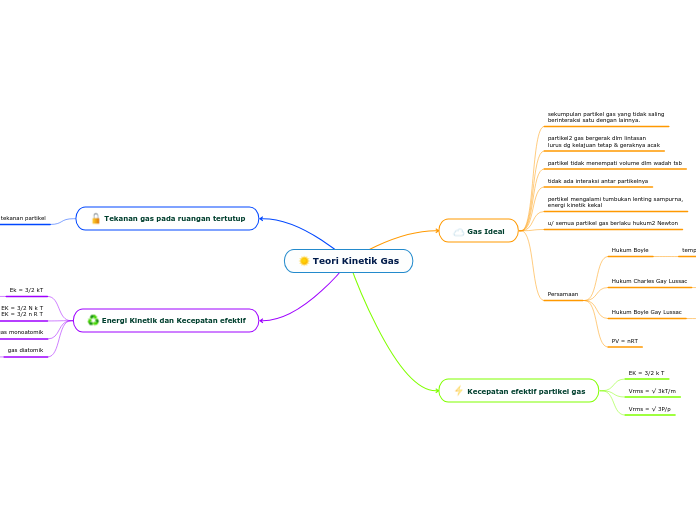
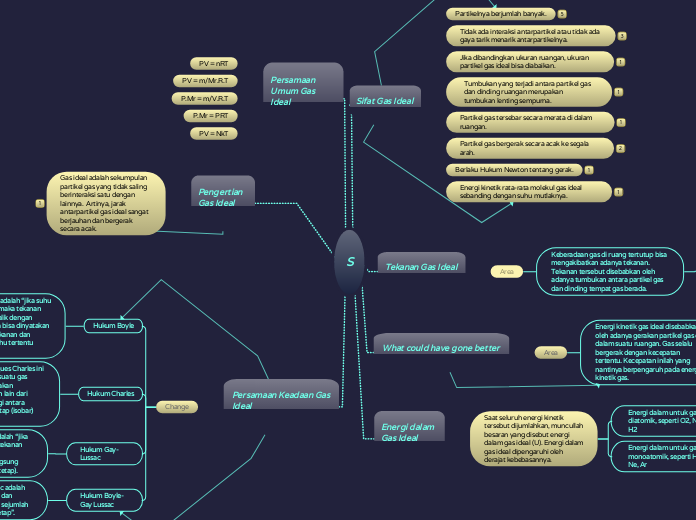
Teori Kinetik Gas
Teori kinetik gas menjelaskan perilaku gas ideal dengan asumsi partikel gas bergerak secara acak dan menempuh lintasan lurus tanpa interaksi satu sama lain. Energi kinetik gas diukur berdasarkan suhu dan jumlah derajat kebebasan partikel, yang berbeda antara gas monoatomik dan diatomik.