realizată de Jeyachandran Pravin (Shss) 3 luni în urmă
86
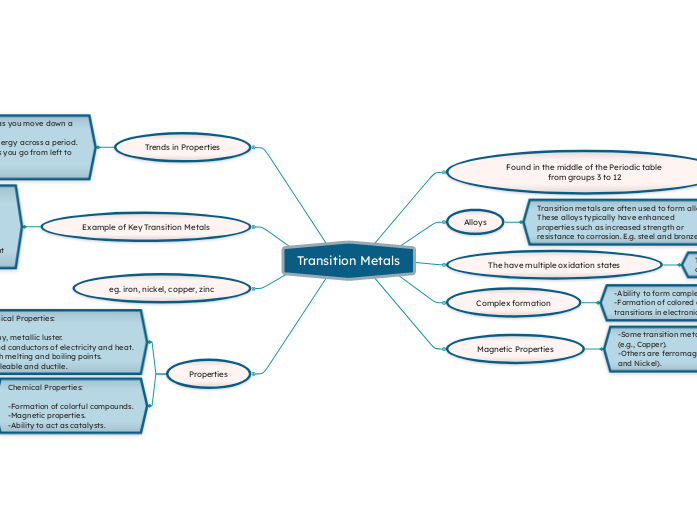
Transition Metals
Transition metals are an essential group of elements located in the middle of the periodic table, specifically in groups 3 to 12. They are known for their ability to form complex ions and colorful compounds, which is attributed to d-d transitions in their electronic levels.