Water Cycle and Changes of State
Steam
Is produced when water boils
Changing States of Water
Condensation
Takes place anytime
Lose heat and changing from gaseous state to liquid state
Boling
Gain heat and changing form liquid state to gaseous state.Takes place at 100 degrees Celcius
Evaporation
Factors
Wind
The stronger it is, the higher the rate of evaporation
Humidity
The higher it is,the lower the rate of evaporation.
Area of exposed surface
The larger it is ,the higher the rate of evaporation
Temperature
The higher it is,the rate of evaporation
Gaining heat and changing from liquid state to gaseous state and takes place at any temperature.
Melting
Gain heat and change from solid to liquid state and takes place at 0 degrees Celcius or higher.
Freezing
Lose heat and change from liquid to solid state.It takes place at 0 degrees Celcius
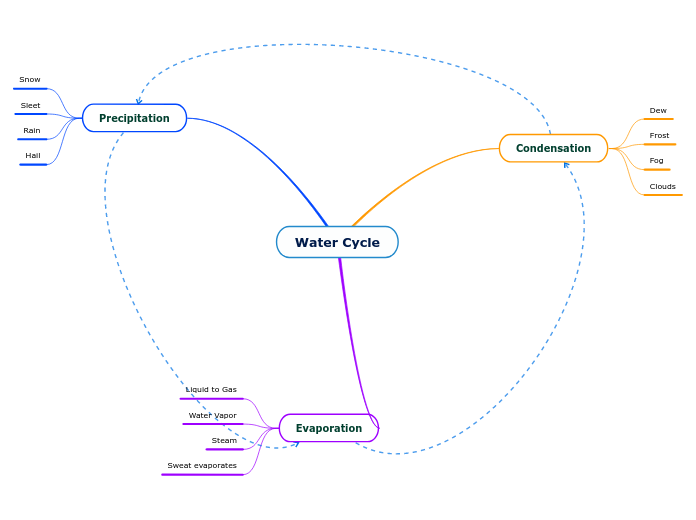
Water is heated up by the sun and rise to form water vapour.This process is called evaporation.Then,the water vapour condenses to form water droplets,.This process is called condensation.After that,they join together to form clouds.They then get heavy and fall as hail,rain,sleet or snow-depending on the temperature.This process is called precipitation.They then go back into the water bodies or soak into the ground .This process is called collection.Then ,the water cycle starts again.
Water
Is the only thing which exists in all three states
Liquid State
Water Droplets
Solid State
Ice,Snow
Visible
Gaseous State
Water Vapour,Steam
Invisible
Covers 2/3 of the Earth`s surface
Comes from Water Bodies
Impurities will lower the freezing point and higher the boiling point.