по Jazmine Smith 3 лет назад
232
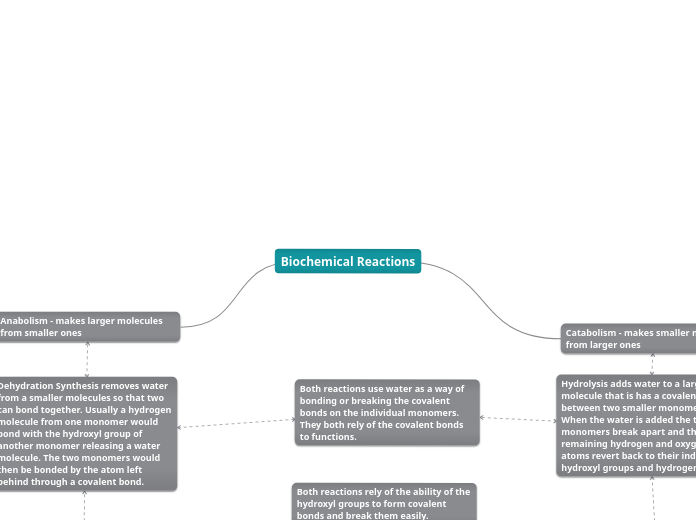
Biochemical Reactions
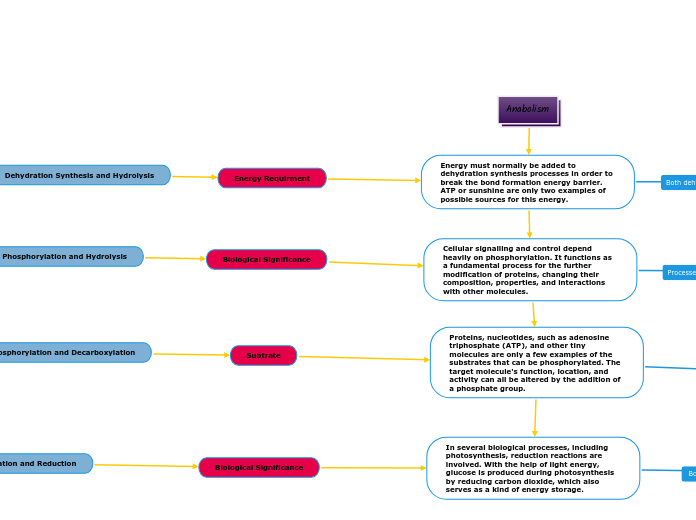
The processes of phosphorylation, decarboxylation, and hydrolysis are fundamental biochemical reactions involving various transformations of molecules. Phosphorylation involves the addition of a phosphoryl group to a molecule, typically transferring from ATP, resulting in a larger, more controlled molecule through covalent bonding.