usage levels <2%
gums & stabilizers
classes of hydrocolloids
cellulose
eg : carboxymethylcellulose (CMC), hydroxypropylmethylcellulose (HPMC)
as thickening, suspending, stabilizing and modifying characteristics
clear solution and stable at pH 4-10
alkaline treatment - converts cellulose to ether
chemically modified
xanthan gum
as thickening agent, suspending & stabilizing effects
pseudoplastic
high stability in heat & pH
completely soluble in cold water & produced high viscosity in low conc.
produced from fermentation of CHO substrate with xanthomonas campestris
guar gum
very stable in pH 4-10
non-gelling gum
more highly substituted than LBG - more soluble in cold water giving high viscosity
locust bean gum (LBG)
as thickening, stabilization of emulsion, inhibition of syneresis, also in canned food, sauces, desserts, beverages, ice-cream, processed meats
cannot form gel by itself, must combine with xanthan gum
insoluble in cold water and must be heated to dissolve again
galactomannan gums - made up of mannose & galactose
from seeds of leguminose
gum arabic
mainly in used in confectionery products, as encapsulation agent, promote foam stabilization in beer, emulsifier in soft drink emulsion
least viscous & most soluble
dissolve easily in hot/cold water
contain small amount of protein, calcium, magnesium and potassium
complex structure - polysaccharide
sap exuded from various species of acacia trees
also known as gum acacia
some applications
in mayonnaise, used propylene glycol alginate
in beverages, eg: dry mix fruit drinks
stabilizing agent in frozen product. used in ice-cream (avoid crystallization)
thermo-irreversible gel
can form gel in cold water in the presence of Ca2+
made up of blocks of :
L-guluronic acid
D-mannuronic acid
derived from brown seaweed
carrageenan
during low concentration, k-carrageenan & i-carrageenan = thermoreversible gels and form double helix, lambda-carrageenan = not form gels and no helix formation
types:
iota (i-carrageenan)
lambda carrageenan
kappa (k-carrageenan)
structure composed of galactan polysaccharides (have sulfate content 15-40%)
highly refined extract of seaweed (rhodophyta family)
pectin
types of pectin
low methoxyl pectin (LMP)
thermoreversible
used as a thickening agent in yogurt fruit and bakery jams
form gels in the presence of Ca2+, with low solids content and wide pH range (1-7)
high methoxyl pectin (MHP)
gels at high solid & low pH
thermally irreversible
firm & short structure, clear & transparent, excellent flavor release
Derived from :
sunflower heads
sugar beet
apple pomace
peel of citrus fruits
natural form called protopectin (insoluble)
gelation of hydrocolloids
thermally irreversible gelling agents
HM pectin
konjac
starch
alginate
thermoreversible gelling agents
HPMC
methyl cellulose
gellan gum
LM Pectin
i-carrageenan
k-carrageenan
agar
gelatin
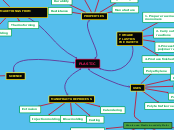
factors that affect gum properties
distribution of side chains
number of side chains
type of side chains
monosaccharide composition
molecular weight
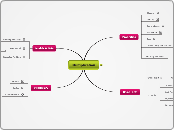
functions
film formation
encapsulation
control of crystallization
suspensions of particulates
emulsion stabilization
gelling or texturizing agents
thickening agents