по Juan Carlos López Valle 3 лет назад
188
Lopez_Valle_Activity6
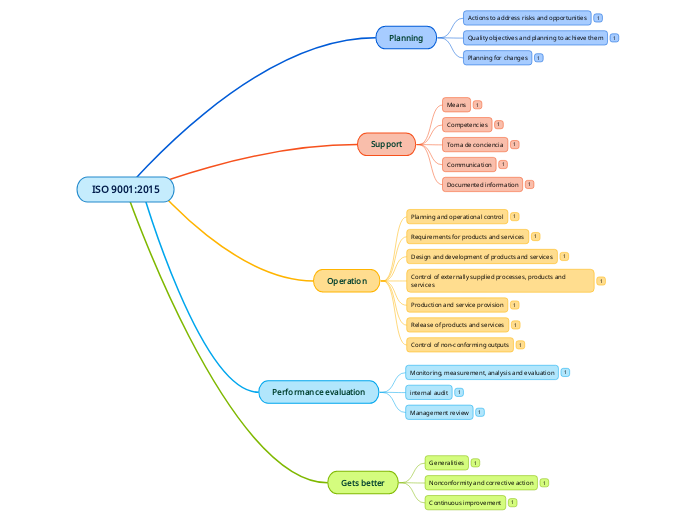
Organizations must address nonconformities, including those from complaints, by taking actions to control and correct them, while also dealing with their consequences. This involves evaluating and eliminating the causes of nonconformities through review and analysis, and implementing necessary actions to ensure similar issues do not recur.