Bio 311c group 4 final Map
Map 3
Map 2
Cell Membranes
Membrane Proteins and R-Groups Orientation
Protein Structure Bonds
Multiple Polypeptides Interacting
Hydrophobic Interactions
Disulfide Bridges
β-Sheets
α-Helices
Hydrophobic R-Groups
Embedded in Lipid Bilayer
Hydrophilic R-Groups
Face Aqueous Exterior/Interior
Structure and Function
Membrane Fluidity
Unsaturated Fatty Acids
Saturated Fatty Acids
Selective Permeability
Large and Polar
Require Transport
Small Nonpolar Molecules (O₂, CO₂)
Pass Freely
Membrane Proteins
Peripheral (Surface Attached)
Integral (Embedded)
Phospholipid Bilayer
Hydrophobic Tail
Phosphate and Glycerol
Communication and Signaling
Cell signaling
Phosphodiesterase
Converts cAMP to AMP
Adenylyl Cyclase
Converts ATP to cAMP
Phosphotase
Removes a phosphate group from proteins
Kinase
Catalyze the transfer of phosphate groups from ATP to proteins
Transduction
Phosphorylation Cascade
Kinases are activated by the addition of a phosphate group
cAMP
Binds and activates protein kinase which goes on to activates other kinases
Reception
Intracellular receptors
Steroid hormone receptor
Membrane receptors
Hydrophilic signal
Ion channel receptor
Signal molecule binds to the receptor and the gate allows specific ions through a channel in the receptor
G protein linked receptor
Signal binds to GPCR causing a change in GCPR shape allowing G protein to bind to it. GDP is replaced with GTP on the G protein. The activated G protein can now activate a nearby enzyme.
Cell communication
Types of signaling
Long distance
Local signaling
Physical Contact
Cell surface proteins
Gap junctions
Energy Transfer
Enzymes
Function
Allosteric Regulation
Feedback Inhibition
Cooperativity
Inhibitors
Activators
Noncompetitive Inhibition
Competitive Inhibition
Normal Binding
Enzyme Activity
Substrate Concentration
Catalytic Cycle
Binding of Substrate
Lowers Activation Energy
Energy Changes
Powered by ATP
ATP Cycle
Mechanical
Transport
Energy Coupler
No Change (ΔG=0)
Exergonic (ΔG0)
Thermodynamics
Laws
2 - Energy transfer increases entropy
1 - Energy can be transferred, but not created/destroyed
Surroundings
System
Open Sytem
Closed System
Metabolic Pathways
Anabolic Pathways
Photosynthesis
Polymerization
Biosynthetic Pathways
Catabolic Pathways
Cellular Respiration
Glucose oxidized, Oxygen Reduced
Anaerobic(doesn't require O2)
fermentation
Lactic Acid Fermentation
Outputs: Lactate, NAD+
Inputs: 2 Pyruvate, NADH
Alchohol fermentation
outputs: ethanol, NAD+
inputs: 2 Pyruvate, NADH
Glycolysis (info in aerobic section)
Aereobic(requires Oxygen)
Oxidative Phosphorylation
Paired Process
Chemiosmosis
H+ transported down concentration gradient using ATP Synthase (facilitated diffusion), produces lots of energy which is used for Pi+ADP=ATP
Electronic Transport Chain
As electrons are transferred down ETC, the energy released is used to pump H+ against concentration gradient
Outputs: H2O, 26-28 ATP
Inputs: O2, 10 NADH, 2 FADH2
Takes place in inter membrane space
krebs cycle
Step 3: Isocitrate is oxidized to alpha ketoglutarate while NAD+ is reduced to NADH
Step 1: Acetyl CoA adds its 2 Carbon groups to Oxaloacetate, forming Citrate
Outputs: 6 NADH, 2 FADH2, 2 ATP
Inputs: 2 Acetyl CoA
Takes place in mitochondrial matrix
Pyruvate Oxidation
Takes place in Cytosol then mitochondrial matrix
Outputs: 2 Acetyl CoA , 2 NADH
Inputs: 2 pyruvate, 2 CoA
Glycolysis
Step 3: Phosphofructokinase converts Fructose 6 Phosphate to Fructose 1,6 Biphosphate
Step 1: Hexokinase converts Glucose to Glucose 6 Phosphate
Uses Substrate level Phosphorylation to produce ATP
Outputs
Total: 2 Pyruvate, 2 NADH, 4 ATP
Net: 2 Pyruvate, 2 NADH, 2 ATP
Inputs: 1 Glucose, 2 ATP
Takes Place in Cytosol
Map 1
Water
Water molecules & heat
Heat is released
Hydrogen bonds form
Heat is absorbed
Hydrogen bonds break
Bonds
Molecular Structure
Bent
Molecules interacting with water
Hydrophilic
Acids and Bases
pOH
Neutral
Water Properties
Universal Solvent
Denser a Liquid than a Solid
Expansion Upon Freezing
High Heat of Vaporization
Evaporative Cooling
High Specific Heat
Helps moderate temperature
Adhesion
Cohesion
Surface Tension
Water Transport in Plants
Chemical Bonds
Electronegativity
Bond Strength
Intramolecular Forces
Ionic
Covalent
Polar Covalent
Non Polar Covalent
Intermolecular Forces
Ion-Dipole
Dipole-Dipole
Hydrophobic
Hydrogen Bonding
Biological Molecules
Nucleic Acids
Nucleosides
Nucleotides
Use Phosphodiester Bond
Nitrogenous Base
Guanine
Adenine
Cytosine
Phosphate Group
Deoxyribose Sugar
Both
Sugar-phosphate Backbone
Base Pairing
Use Hydrogen Bonds
RNA
Single-Stranded
Uracil
Deoxyribose
DNA
Complementary Base Pairing
Uses Hydrogen Bond
Double-Stranded
Thymine
Ribose
Carbohydrates
Glucose
Beta
Alpha
Polysaccharides
Use Glycosidic Linkage
Bet 1, 6
Beta 1, 4
Alpha 1, 6
Alpha 1, 4
Storage
Starch
Amylopectan
Some Branching
Amylose
Glycogen
Extensive Branching
Structure
Cellulose
No Branching
Linear
Lipids
Steroids
Cholesterol
LDL
HDL
In Cell Membrane
Amphipathic
Phospholipids
Parts
Hydrophobic Tails
Hydrophilic Head
Form Closed Liquid Bilayers
Fat Molecule
Uses Ester Linkage
Fatty Acids
Saturated
Solid at Room Temperature
Unsaturated
Double Bond
Isomers
Cis
Trans
Liquid at Room Temperature
Glycerol
Protiens
Protien Structure
Physical/Chemical Conditions
Solution Prevents Disulfide Bond
Salt
Temperature
pH
Amino Acid Sequence
Protien Folding
Quaternary
Interchain Interactions
Tertiary
Interaction of R-Group
Disulfide Bonds
Van Der Waals
Ionic Bonds
Secondary
Types
Beta Pleated Sheets
Alpha Helices
Hydrogen Bonds
Primary
Peptide Bonds
Dehydration Synthesis
Amino Acid
Side Chain (R-Group)
Acidic
Basic
Nonpolar
Polar
Carboxyl Group
Amino Group
Main Chain
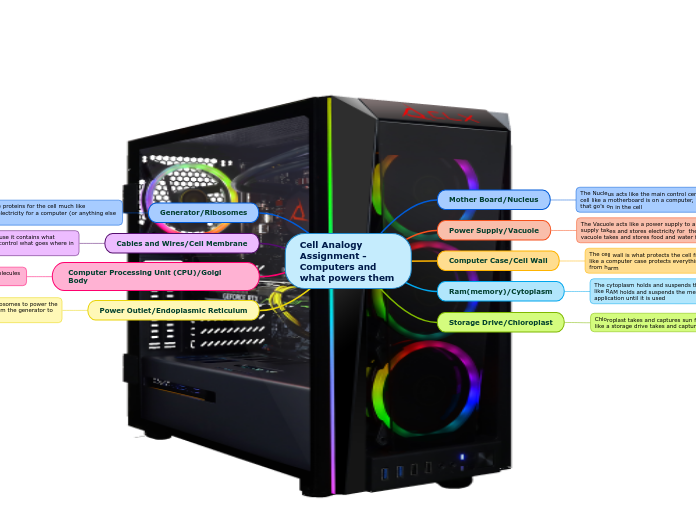
Cellular Functions and Organelles
Prokaryotic Only
Plant Only
Chloroplast
Central Vacuole
Cell wall
Plasmodesmata
Present in both Animal and Plant Cells
Cytoskeleton
Intermediate Filaments
Microfilaments
Microtubules
Peroxisome
Lysosome
Golgi Apparatus
Endoplasmic Reticulum
Smooth ER
Rough ER
Ribosomes
vacuoles
Nucleus
Nuclear envelope
Vesicles
Mitochondria
Animal Only
Gap Junction
Desmosome
Tight Junction
Extracellular Matrix
Integrins
Protoglycan
Fibronectin
Collagen