av yeo peifang för 18 årar sedan
330
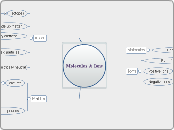
Molecules & Ions
av yeo peifang för 18 årar sedan
330

Mer av detta
Made up of atoms of the same type
Produced by chemical reaction
Constant constituent
Different from those of its constituent elements
Made up of atoms of different type
Example: Ink
Example: Salad
Different number of neutrons
Same number of protons