Inhibition of Bile Acid Synthesis
LXR
Activation by excess lipids
CYP8B1
CYP7B1
HSD3B7
AKR1C4
AKR1D1
Cholic Acid
Chenodeoxycholic Acid
CYP27A1
CYP7A1
Treatment with PPARa agonists: improved cell anatomy and biochemical parameters.
Research suggests: SREBP-related links between fatty acid and glucose metabolism are relevant for type 2 diabetes
No approved surgical or drug therapy for NFLD.
Cell therapies for hepatocytes
The Stages of Liver Damage
Balance the cycle for the uptake of fats, oxidation, and export.
A lot of current treatments are aimed towards obesity and weight loss.
Design then improve technology that will increase enzyme levels to export and breakdown lipids in the liver it. This can reduce inflammation, scarring, and cell death.
Therapeutic advancements
So what can we do?
Our importance and the bigger picture for society.
-Major proteins and enzymes involved in the breakdown of fatty acids in the Burmese Python liver
-Human orthologs
-Better understanding of common liver health problems
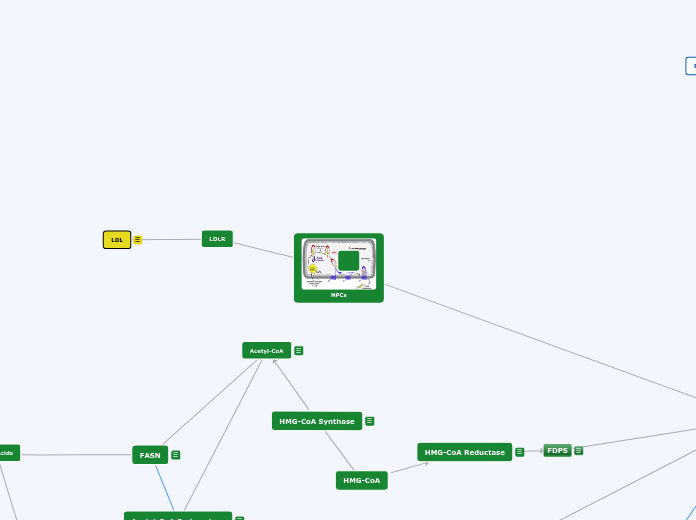
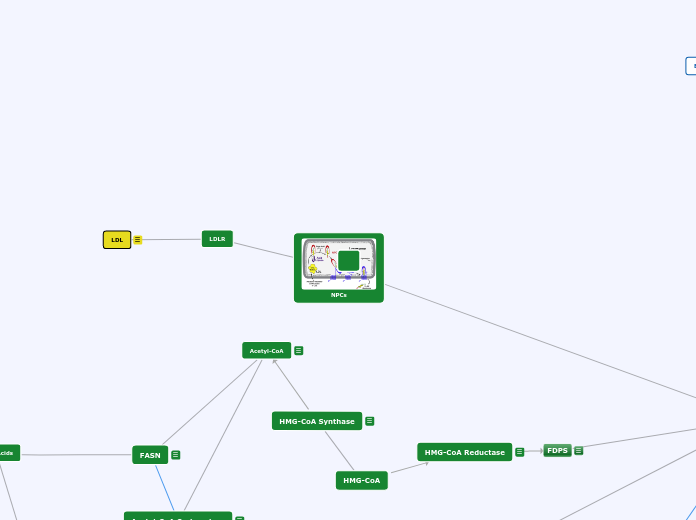
HMG-CoA
HMG-CoA Reductase
Rate controlling enzyme of synthesis of cholesterol from Acetyl-CoA (mevalonate pathway)
***Prime site for regulation***
Negative feedback mechanism--> cholesterol inhibits HMGCR from producing more cholesterol
Target for "statin" drugs (cholesterol reducing drugs)
active when blood glucose is high (indirectly regulated by pancreatic hormones insulin and glucagon)
FDPS
Mevalonate pathway produces two five carbon building blocks (IPP and DMAPP);
FDPS catalyzes the formation of FPP from these building blocks
Through additional subsequent reactions, over 30,000 biomolecules including cholesterol, heme, vitamin K, coenzyme Q10, and all steroid hormones
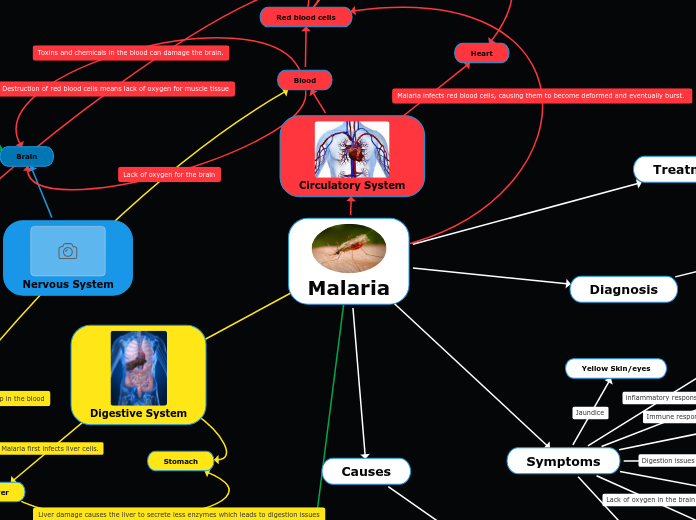
Recycled Bile Acids
CYP3A4
http://www.ncbi.nlm.nih.gov/pubmed/15708356
ACOX1
The first gene involved in the beta oxidation pathway of fatty acids. Cataylzes the desaturation of acyl-CoAs to 2-trans-enoyl-CoAs & produces hydrogen peroxide in the process. Also the rate limiting enzyme for beta-oxidation.
Is a PPARalpha target gene. Metabolizes ligands for PPARalpha.
PPARalpha can induce ACOX1 for peroxisomal fatty acid oxidation
Has 2 isoforms that function as a heterodimer.
Knockout of ACOX1 doesn't effect bile acid synthesis
Plays major role in liver metabolism and the development of a fatty liver.
Part of a feedback loop for the expression of peroxisomal beta-oxidative genes.
***Inhibits PPAR alpha by degrading ligands such as HETEs Acox1 is also involved in Beta-Oxidation pathway converting Acetyl-CoA into Enoyl-CoA***
PLTP
PLTP functions to stabilize the formation of Apolipoprotein B containing lipoprotein within the liver (conjugates to LDL) and stabilizes the export of Apolipoprotein A (conjugates to HDL) from the liver. It has additional functions in the plasma of HDL conversion.
SCD5
SCD in general is a rate limiting step in the process of creating Monounsaturated fatty acids, also helps create phospholipids, helps generate Fatty acyl CoA, which will then be eventually turned into Tri-acyl-glyceride, which is the primary unit for storage of fat in animals and humans.
OATP1B3
OATP1B3 is a non-Mg dependent membrane transport protein found on the surface of hepatocytes. It functions to bring secondary bile acids back into the liver for recycling. When it is knocked out, a build up of bile acids in the portal vein indicates an inability for the bile acids to re-enter the liver.
***recycled bile acids can serve as ligands for FXR which dimerizes with SHP and RXR to suppress the expression of Cyp7a1 a key step in bile acid synthesis pathway***
So OAT may help inhibit the process by promoting the import of circulating Bile Acids
FASN
- Last step in de novo lipogenesis
- Produces FAS (Fatty Acid Sythase) which functions to convert acetyl-CoA and malonyl-CoA into long-chain saturated fatty acids
- Transcriptionally regulated by SREBP-1c
-Agonistic relationship with PPARalpha
Acetyl-CoA Carboxylase
SREBP-1c activate expression of 30 genes to synthesis cholesterol,fatty acids, and triglycerides
Acetyl CoA is carboxylated with Acetyl CoA carboxylase. Forming malonyl CoA.
-Which synthesize Fatty acids
HMG-CoA Synthase
-Segment of the cholesterol biosynthesispathway
-Acetyl-CoA ->HMG-CoA
-regulation is coordinated with HMG-CoA reductase
- cholesterol inhibits HMGCR from producing more cholesterol
- active when blood glucose is high
Acetyl-CoA
· Fatty Acids are converted into Acetyl-CoA by beta oxidation
· Acetyl-CoA is converted into 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). Rate limiting step
FGFR1
FGF21
FGF21 is a signaling molecule that binds to the cellular receptor FGFR1 in hepatocite and adiposite tissue. It is activated by PPARalpha signaling in the starvation response pathway in mice. In mice FGF21 is responsible for autocrine, endocrine, and paracrine signaling that results in the lipolysis of WAT and production of ketone bodies.
***We recently found that the hepatic gene expression of FGF21 is induced 2- to 4-fold following pharmacological or genetic interruption of bile acid circulation, and that feeding a sucrose-rich diet induces hepatic FGF21 expression remarkably by 25-fold***
Has triglyceride lowering effects
Tentative PPARα responsive elements (PPREs) were present in the promoter regions of both mouse and human FGF21 genes
PPARgamma
Type 2 nuclear receptor, localized in the nucleus and forms a heterodimer with RXR to activate genes as a transcription factor. Ligands and coactivators include oxidized fatty acids and molecules generated in intermediary metabolic pathways. Activates genes that cause: differentiation of cells into adipocytes, intake of cholesterol and fatty acids, and promotes transcription of glycolytic enzymes in the liver. Essentially functions as a metabolic sensor that regulates gene expression in response to the metabolic needs of the cell.
PPARalpha
- Belongs to the type II nuclear receptor family, localized in the nucleus
- Expression levels are highest in tissues w/ active FA catabolism such as the Liver
- PPAR alpha endogenous ligands: FA's and FA derivatives such as TCA cycle precursor Acetyl-CoA
- Upon ligand binding PPAR alpha
- Enzymes such as FAS FACS are required for ligand generation
- Prior to ligand binding PPAR alpha heterodimerizes with NCOR and SMRT corepressors
- PPAR alpha requires PBP/MED1 coactivator and RXR alpha for transcription
- PPAR alpha influences the expression of hepatic lipogenic genes by regulating SREBP-1c and LXR alpha
*PPAR alpha senses lipid influx and promotes the break down into Acetyl-CoA to prevent steatosis
FA's bind to PPAR alpha--> Activates lipogenic genes and upregulates all 3 fatty oxidation systems----> Acetyl-CoA---> Precursor of Cholesterol synthesis---> *aditionally Acetyl-CoA is also a ligand of PPAR alpha
***. Since PPARα stimulation also reduces bile acid synthesis [11] and [12] and plasma triglycerides [11],[13] and [14] we speculated that PPARα may be involved in the regulation of the expression of hepatic FGF21***
PPARalpha also promotes the transcription of Fatty Acid Translocase CD36 to bring FA's into the hepatocyte for break down. Also has been shown to regulate the expression of Cyp7a1 which the rate limiting enzyme in the synthesis of bile acids from cholesterol.
There was in increase of gene expression for the regulation of Bile Acids at 1dpf and 3dpf
The presence of cholesterol after feeding, up regulates proteins involved in bile synthesis so cholesterol can be excreted, seen at 1dpf. When cholesterol is being excreted and levels are decreased, the proteins are down regulated, that is seen at 3dpf.
There was an increase of gene expression for export of Bile Acid at 1dpf and 3dpf
There was an increase of gene expersion for synthesis of Bile Acid at 3dpf.
There was an increase of gene expression for import of lipids seen at 1dpf.
Regulation of Bile Acid
Export of Bile Acids
Synthesis of Bile Acid
Import of Lipids
Duplicate results
Used both experimental and control (18s or Tubulin) primers
Test with PCR
Test with gel electrophoresis/spectrophotometry
Looked for 18s subunit bands (all other RNA too small to seeon gel)
In silico & in vitro Validation
qPCR
cDNA Synthesis
synthesized cDNA from 3 different time points: fasted, 1dpf,3dpf, & 10dpf
Liver RNA isolation
Primer Design
Designing primers over an intron
~20bp
Appropriate GC content, melting temp, etc.
Python genome is unannotated
Experimental Design
Excreting excess fats from serum
Gene expression changes to aid digestion of excess fats
Interconnected nature of the bile acid pathway
Genes expression must reflect how the snake isable to deal with the influx of fat in the system.
Predictions
Gene expression changes?
Many genes involved in the process of bile production and excretion.
How is the regulation of these genes affected when an influx of fat comes into the system?
Mechanism to eliminate excess fat?
Fat Digestion
Bile Acid Synthesis
Synthesized from lipids through two main pathways
Aids in digestion and absorption of fats
Without bile, fats cannot be digested
95% of bile acids are recovered from the intestines to be brought back to liver
Many genes involved, redundancy (at least in humans), and high levels of regulation
Serum Lipid Levels
Serum triglycerides and fatty acids peak at 1dpf and return to normal by 6-10dpf
At 1dpf the serum of the python becomes milky white
When these serum fatty acids administered to mouse cells, cardiac hypertrophy and liver hyperplasia were observed
Liver Hyperplasia
Liver increases up to 100%
Cells dividing, not getting bigger
Questions
Postprandial Physiological Changes
Python coordinates feeding and fasting responses across feeding and tissues--a truly integrated response
Feeding
A snake can consume a meal up to 1.6 times its own size
Comparable to a 145lb person eating a 232lb meal
Digestion completes by 6 to 10 days after feeding
Burmese Python
Extreme model for physiologic regulation among vertebrates
Results & Conclusions
Introduction
ABCG1
FXR
Farnesoid X Receptor (FXR) is a nuclear receptor produced in the liver and intestines. After being bound by a bile acid ligand, FXR often dimerizes with RXR or SHP where the complex moved to the nucleus to regulate gene expression. A main role of FXR activation is to supress CYP7A1 in it's role of converting cholesterol to bile acids.
RXR
ABCC2
BSEP
Export to Intestines
Cholesterol
-In humans, the most heavily utilized pathway of cholesterol processes involves the metabolism of cholesterol into bile acids.
-Predominantly CA and CDCA
-Bile acids are conjugated to amino acids to produce bile salts which then assist in the solubilization, transport and removal of cholesterol.
-In the context of the python and maintaining cholesterol homeostasis, excess cholesterol is toxic and must be removed, facilitating the essentiality of this pathway.
Bile Salts
-Again, the essentiality of the production of bile salts cannot be stressed enough. The only mechanism of cholesterol removal is through its solubilization which is facilitated by bile salts.
ABCA1
ABCA1 and ABCG1
· Members of the superfamily of ATP-binding cassette transporters
· Function: efflux of cholesterol from cells in the form of HDL
· Play a role in the current model for reverse cholesterol transport
SR-B1
SRB1 is the protein encoded by SCARB1 gene.
Facilitates uptake of cholesterol esters from high density lipoproteins into the liver.
Helps to drive the clearing of serum.
CD36
CD36
· Class B Scavenger Receptor Protein
· Functions as a translocase facilitating the uptake of lipids
· Main function in the liver is the uptake of long chain fatty acids
LDLR
HDL
Fatty Acids
LDL
(LDL) carry cholesterol to all cells in your body, including arteries that supply blood to your heart. LDL cholesterol is sometimes called bad cholesterol because it build up in walls of your arteries.