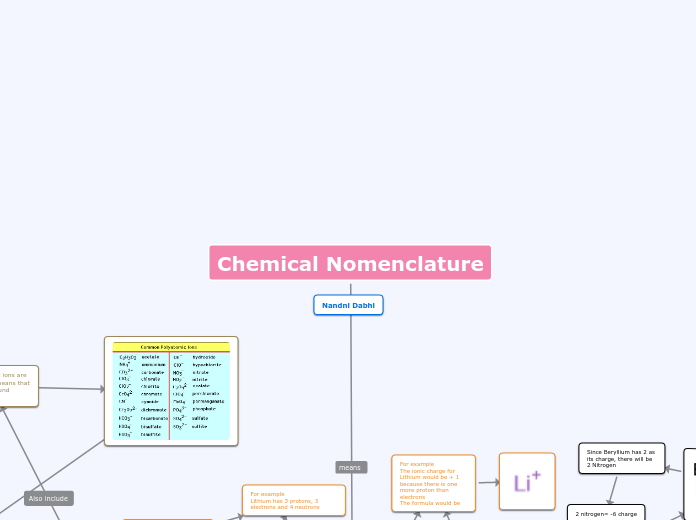
Polyatomic ions are ions made of more than one atom. Carbonate CO3, Nitrate NO3, Phosphate PO4, Sulphate SO4, Hydroxide OH, Chlorate ClO3, Hydrogen Carbonate HCO3, Ammonium NH4.
To write the Name first write the metal, then write the polyatomic ion.
To write the formula first, determine whether it is ionic, multivalent or polyatomic. Then, write the symbols and the charges. Next criss-cross the charges and put brackets if needed. Finaly reduce it to the lowest ratio.
To write the formula of multivalent ionic compounds first, write the symbols of each element. Then, write the charges above the symbol remember to use brackets. Next, criss-cross the charges and reduce them to the lowest ratio.
Some metals can lose different amount of electrons having more than one charge these are called multivalent. To write the names of multivalent ionic compounds first make sure it is ionic, then make sure it is multivalent. Next, uncross the subscripts to find the charge of each element, next check the charge of the elements to make sure they are correct and unreduce them. Then, write the name of the metal and the charge of the metal in roman numerals in brackets. Then, write the non-metal ending with ide
Binary ionic compounds are made of a metal and a non-metal. They are solid at room temprature, exist as crystal lattices, and the chemical formula is written using the lowest ratio. The first part of the name is always the metal and the second is always the non-metal with the suffix ide. To write the chemical formula of a binary ionic compound first make sure that the compound is ionic, then write the symbols and the charge of the elements, next criss-cross the charge to make the subscript and reduce to the lowest ratio. To name them first make sure that it is ionic, then write the metal first, then write the non-metal ending in ide.
Bases are ionic compounds of metals and hydroxide ions. To name a base first write the first element and then write hydroxide. Some bases have common names such as: NaOH= Baking soda, Mg(OH)2= milk of magnesia, Ca(OH)2 (aq)= lime water. To write the chemical formula of a base first determine the cation and anion(OH), then determine the charge of the cation and determine how many OH is needed to make the whole charge 0
A base is a compound that produces hydroxide ions[OH (aq)] when dissolved in water. Some are safe to eat (baking soda, antacids), and others are dangerous. Bases have a slippery feel and a bitter taste.
A base is a compound that produces hydroxide ions[OH (aq)] when dissolved in water. Some are safe to eat (baking soda, antacids), and others are dangerous. Bases have a slippery feel and a bitter taste.
An acid is a compound that produces Hydrogen ions when it dissolves in water. Some acids are sour and safe to eat (i.e. acetic acid, lemons). Other acids are corrosive and dangerous. There are two types of acids, binary acids (H + ion. hydro[root]acid ) and oxyacids.(one or more H + polyatomic ion). Oxyacids are acids that have polyatomic ions containg oxygen. (HNO3, H2C03, HCl03, H2SO4, H3PO)
Make sure the compound is molecular.
Determine the prefix for each element.
Write the name and prefix for each element.
Do not use the prefix Mono- for the first element.
Make sure the compound is molecular and not ionic or polyatomic.
Write the symbols for each of the elements.
Write the subscripts for each of the elements using the prefixes.
Do not reduce.
Made of 2 or more non-metals.
They share electrons instead of giving or taking.
Use prefixes to know how many atoms the element has
Types of Compounds
Acids bases
Bases
Naming and Chemical Formula
Acid
Ionic
Binary
Multivalent
Molecular
Naming Molecular Compounds
Writing the Formula of Molecular Compounds
Polyatomic
Name
Formula