Catabolism
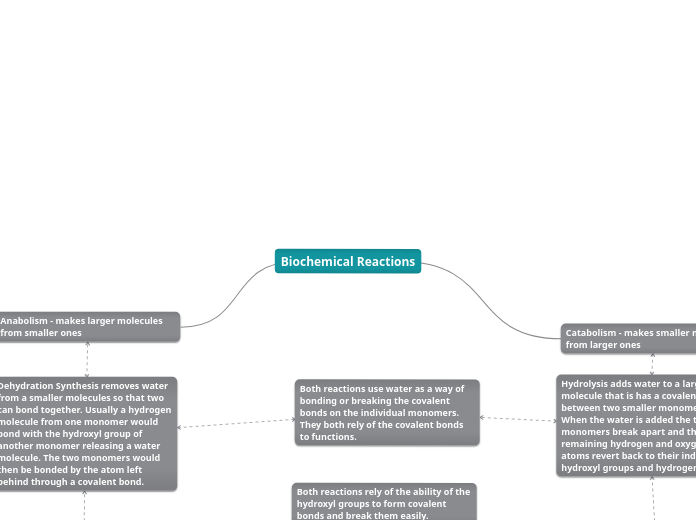
Energy is released when When bonds are destroyed during hydrolysis processes, During the hydrolysis process, the energy that was once held in the bigger molecule's chemical bonds is released.
Hydrolysis is a common process in biological processes. Because it simplifies complex molecules into ones the body can absorb and use,it is essential to how food is digested. Hydrolysis is involved in the breakdown of macromolecules such asproteins, lipids, and carbohydrates,in addition to recycling cellular components.
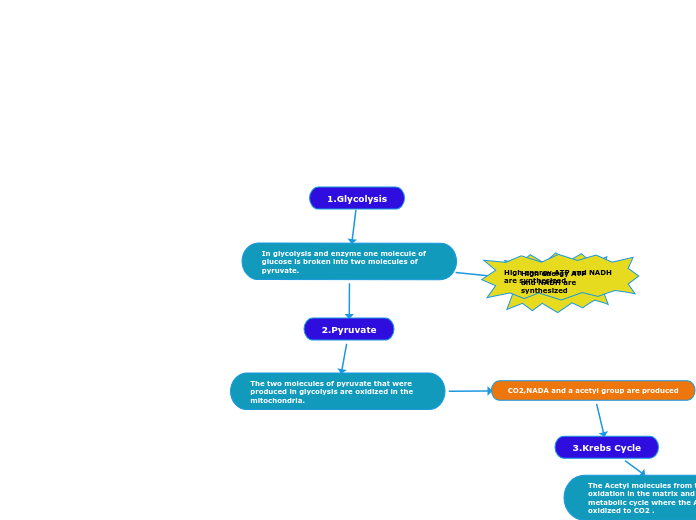
Decarboxylation normally involves eliminating a carboxyl group from organic acids, including pyruvate, amino acids, fatty acids, and organic acids generated from the citric acid cycle. As a byproduct of the decarboxylation process, carbon dioxide is produced.
Oxidation reactions are frequently linked to activities that transfer energy, including cellular respiration. Oxidation processes are crucial for the synthesis of molecules full of energy,like ATP,in biological systems.
Anabolism
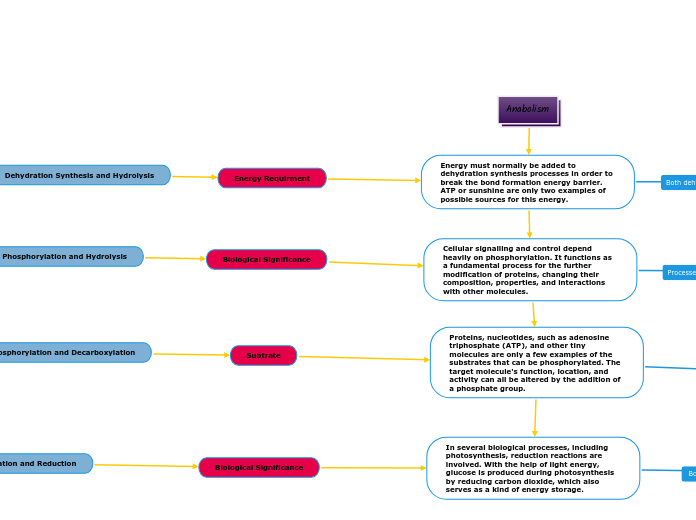
Energy must normally be added to dehydration synthesis processes in order to break the bond formation energy barrier. ATP or sunshine are only two examples of possible sources for this energy.
Cellular signalling and control depend heavily on phosphorylation. It functions as a fundamental process for the further modification of proteins, changing their composition, properties, and interactions with other molecules.
Proteins, nucleotides, such as adenosine triphosphate (ATP), and other tiny molecules are only a few examples of the substrates that can be phosphorylated. The target molecule's function, location, and activity can all be altered by the addition of a phosphate group.
In several biological processes, including photosynthesis, reduction reactions are involved. With the help of light energy, glucose is produced during photosynthesis by reducing carbon dioxide, which also serves as a kind of energy storage.
Oxidation and Reduction
Subtrate
Phosphorylation and Decarboxylation
Biological Significance
Phosphorylation and Hydrolysis
Energy Requirment
Dehydration Synthesis and Hydrolysis