作者:Gabriela Segovia 2 年以前
106
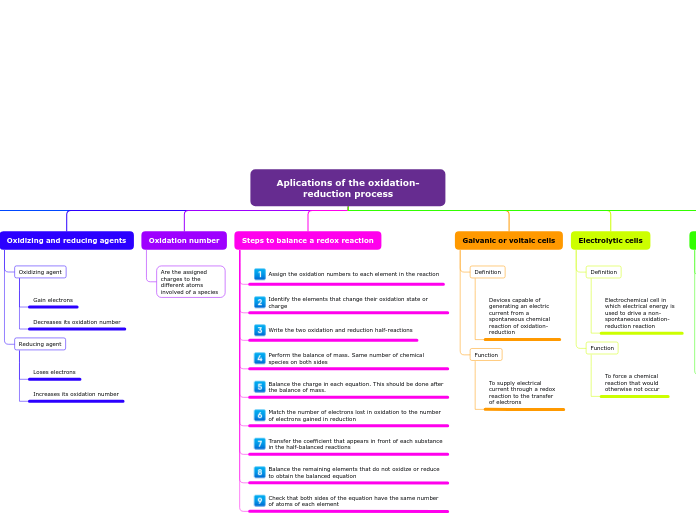
Aplications of the oxidation-reduction process
Galvanic cells, also known as voltaic cells, are devices that generate electric current from a spontaneous oxidation-reduction chemical reaction, supplying electrical current through the transfer of electrons.