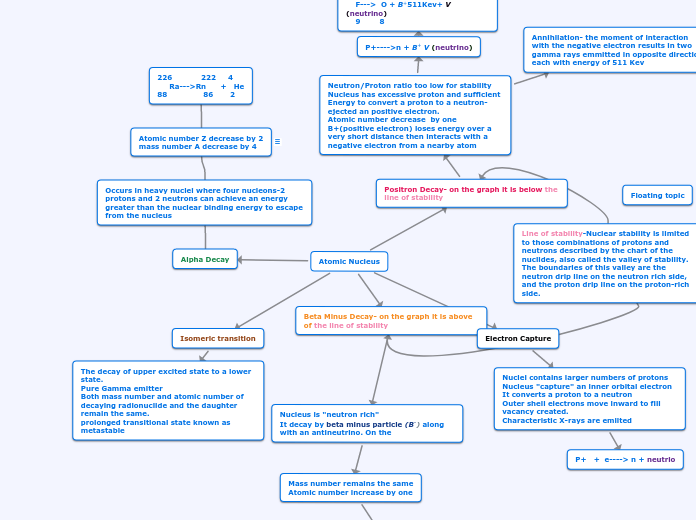
Floating topic
Line of stability-Nuclear stability is limited to those combinations of protons and neutrons described by the chart of the nuclides, also called the valley of stability. The boundaries of this valley are the neutron drip line on the neutron rich side, and the proton drip line on the proton-rich side.
Atomic Nucleus
Electron Capture
Nuclei contains larger numbers of protons
Nucleus "capture" an inner orbital electron
It converts a proton to a neutron
Outer shell electrons move inward to fill vacancy created.
Characteristic X-rays are emiited
P+ + e----> n + neutrio
Isomeric transition
The decay of upper excited state to a lower
state.
Pure Gamma emitter
Both mass number and atomic number of decaying radionuclide and the daughter remain the same.
prolonged transitional state known as metastable
Beta Minus Decay- on the graph it is above of the line of stability
Nucleus is "neutron rich"
It decay by beta minus particle (B-) along with an antineutrino. On the
Mass number remains the same
Atomic number increase by one
N----> P + B- + V-(antineutrino)
131 131
I----> Xe + B- + V-(antineutrino)
53 54
antineutrino (v)- is entily almost without mass and charge and is primarily need to conserve energy in the decay.
Positron Decay- on the graph it is below the line of stability
Neutron/Proton ratio too low for stability
Nucleus has excessive proton and sufficient Energy to convert a proton to a neutron- ejected an positive electron.
Atomic number decrease by one
B+(positive electron) loses energy over a very short distance then interacts with a negative electron from a nearby atom
Annihilation- the moment of interaction
with the negative electron results in two gamma rays emmitted in opposite direction each with energy of 511 Kev
P+---->n + B+ V (neutrino)
131 18
F---> O + B+511Kev+ V (neutrino)
9 8
The neutrino- is opposite of antineutrino. It caries away the difference between decay energy and beta energy.
Alpha Decay
Occurs in heavy nuclei where four nucleons-2 protons and 2 neutrons can achieve an energy greater than the nuclear binding energy to escape from the nucleus
Atomic number Z decrease by 2
mass number A decrease by 4

226 222 4
Ra--->Rn + He
88 86 2