Thermochemistry
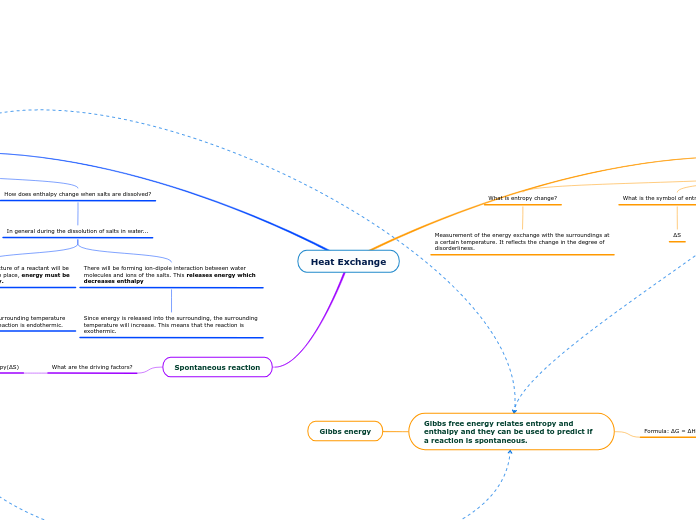
Chemical reactions that involve transfer of heat between system and surroundings
Endothermic
More heat energy is added than what is released
Bond breaking
Exothermic
Bond making
More heat energy is released than what is added
Standard Enthalpy Change
Neutralization
enthalpy change when a strong acid and base react together to form one mole of water under standard conditions
1. Calculate the number of moles of acid and base using n=cv
2. Determine the limiting reactant
3. Add the volumes of acid and base together (where 1cm^3=1g) to get the mass
4. Use Q=mc∆T to calculate enthalpy change
5. Use ∆H=Q/n
Chemical Bonding and Structure
Bond Strength
measure of energy required to break bond
Bond Length
measure of the distance between two bonded nuclei
Covalent Structures
nonmetals (0 ≤ ΔEN ≥ 1.7)
Ionic Bonding
metals and nonmetals (ΔEN ≥ 1.8)
Hybridization
formed when two atomic orbitals, each containing one electron, overlap to from a new combined orbital
Vespr Theory: used to predict 3D molecular geometry based on the atoms/ions valence shell electron bond pairs
Molecular Polarity
partial charge distribution of atoms in a compound is uneven which is dependent on individual molecular geometry and symmetry
Intermolecular Forces
forces that exist between molecules
London Dispersion Forces → all molecules(polar/nonpolar)
Dipole-Dipole/Hydrogen Bonding → polar
Periodicity
Non-Metals
gain electrons in reactions (anions)
Metals
lose electrons in reactions (cations)
Periodic Trends
Melting and Boiling Point
Decreases down a group/ Increases across a period until group 14 and then decreases
Electronegativity
Atomic Radius
Increases down a group/ Decreases across a period
Ionization Energy/ Electron Affinity
Decreases down a group/ Increases across a period
Atomic Structure
Standard Notation
Orbital
Each orbital has a specific energy level, with 1s being the lowest energy level
Hund's Rule: orbitals of same sublevel are filled singly, then paired up
Aufbau Principle: electrons are added to lowest energy level first
Pauli Exclusion Principle: no more than two electrons per orbital and they must spin oppositely
Electron Configuration
Ionization energy: minimum energy required to remove an electron from a neutral gaseous atom/moleule in its ground state
x^(n-1)(g) → x^n+(g) + e⁻
Stoichiometry Relationships
Isotopes: atoms of the same element with different mass numbers, but similar chemical properties
Mass spectrometer
Titrations: technique where solutions of known concentration is used to determine concentration of unknown solution (n=CV)
Limiting Reactant: the first reactant to be consumed completely in a chemical reaction
Excess Reactant: the reactant that could keep reacting if limiting reactant had not been done
Mole Concept
Avogadro's Constant 6.02×10²³ objects/mol⁻¹
Molar Mass (g/mol)
M=m/n
Mole (mol)
n=m/M
n=N/L
Measurement and Data Processing
Accuracy
Systematic Errors: Flaw in the actual experimental design, defect with instrument or the way the measurement was taken
Precision
Random Errors: Uncontrolled variables in an experiment
Scientific Notation: used to make numbers and quantities easier to comprehend and write
Significant Figures: the number of digits reflecting the precision of a given measurement
Graphing Techniques
Lab Equipment
Maahi's Chemistry Connections