The way in which electrons are arranged around the nuclei of atoms.
Arrangement of Electrons in Atoms
SHELLS(n)
SUBSHELLS(i)
ORBITALS (m1)
• Each sublevel contains at least one orbital
– Area of higher probability of finding electrons
– Every orbital holds 2 electrons
– Different sublevels have different shaped
orbitals
• s = spherical
• p = dumbbell
total of 7
– 1st energy level is closest to nucleus
– Contain Each energy level does not contain the same sublevels
– As the distance from the nucleus increases energy levels can hold more electrons –Therefore, they can have more
sublevels
• Four types
–s (lowest energy)
–p
–d
–f (highest energy)
If you want to name a specific sublevel in an energy level,
you write the energy level number followed by the sublevel.
Example: 3rd energy level, d sublevel is written as 3d.
Energy Level, n # of sublevels Letter of sublevels # of orbitals per sublevel# of electrons in each orbital Total electrons inenergy level
1 1 s 1 2 2
2 2 s p
1
3
2
6 8
3 3
s
p
d
1
3
5
2
6
10
18
4 4
s
p
d
f
1
3
5
7
2
6
10
14
32
• First, determine how many electrons are in the
atom. Iron has 26 electrons.(nutral atom electrons and atomic number/#protons is the same)
• Arrange the energy sublevels according to
increasing energy:
–1s 2s 2p 3s 3p 4s 3d ...
• Fill each sublevel with electrons until you have
used all the electrons in the atom:
–Fe: 1s2 2s2 2p6 3s2 3p6 4s2 3d 6
• The sum of the superscripts equals the atomic
number of iron (26)
An excited atom has an electron or electrons which are
not in the lowest energy state. Excited atoms are
unstable energetically. The electrons eventually fall to a
lower level. * is used to indicate an excited atom. For
example: *Li 1s2 3p1. (The ground state for Li is 1s2 2s1.)
• Write an excited electron configuration for the following
atoms.
• *Al
• *K 22
• Write the electron configuration of the neutral atom.
• Remove electrons from the orbital with the highest principal QN (value of n)
– Fe [Ar] 4s2 3d6
– Fe2+
• A. Fe [Ar] 4s2 3d6
• B. Fe [Ar] 3d6
• C. Fe [Ar] 4s2 3d4
• D. Fe [Ar] 4s1 3d1
• Write a ground state electron configuration for a neutral atom
K
Ne
• Write a ground state electron configuration for
these ions.
O2-
Na+
21
– K+
– As3+
Three rules—the aufbau principle, the Pauli
exclusion principle, and Hund’s rule—tell you how to find the electron configurations of atoms.
• Aufbau Principle
– According to the aufbau principle, electrons occupy the
orbitals of lowest energy first. In the aufbau diagram
below, each box represents an atomic orbital.
• Pauli Exclusion Principle
– According to the Pauli exclusion principle, an atomic
orbital may describe at most two electrons. To occupy the
same orbital, two electrons must have opposite spins; that
is, the electron spins must be paired.
• Hund’s Rule
– Hund’s rule states that electrons occupy orbitals of the
same energy in a way that makes the number of electrons
with the same spin direction as large as possible.
• We use a numbering system to indicate
electrons in an atom
• Its given first by a number: 1,2,3,4, etc...
dictated by the period number electron shell
• Then follows a lower case letter: s,p,d, f...
dictated by the group from the periodic table orbital type
• Then follows a superscript given over the letter: indicates number of electrons in that orbital
• The electron configuration of an atom is a shorthand method of writing the location of
electrons by sublevel.
• The sublevel is written followed by a superscript with the number of electrons in the sublevel.
– If the 2p sublevel contains 2 electrons, it is written 2p2
The periodic table can be used as a guide for electron configurations.
• The period number is the value of n.
• Groups 1A and 2A have the s-orbital filled.
• Groups 3A - 8A have the p-orbital filled.
• Groups 3B - 2B have the d-orbital filled.
• The lanthanides and actinides have the f-orbital
filled. We can use the periodic table to predict which
sublevel is being filled by a particular element.
Noble Gas Core Electron Configurations
• Recall, the electron configuration for Na is: Na: 1s2 2s2 2p6 3s1
• We can abbreviate the electron configuration by indicating the innermost electrons with the symbol
of the preceding noble gas.
• The preceding noble gas with an atomic number less than sodium is neon, Ne. We rewrite the electron configuration:
Na: [Ne] 3s1
Condensed Electron Configurations
• Neon completes the 2p subshell.
• Sodium marks the beginning of a new row.
• So, we write the condensed electron configuration
for sodium as Na: [Ne] 3s1
• [Ne] represents the electron configuration of neon.
• Core electrons: electrons in [Noble Gas].
• Valence electrons: electrons outside of [Noble Gas].
Electron Configurations
Exceptional Electron Configurations
Some actual electron configurations differ from
those assigned using the aufbau principle
because half-filled sublevels are not as stable as
filled sublevels, but they are more stable than
other configurations. • Exceptions to the aufbau principle are due to
subtle electron-electron interactions in orbitals
with very similar energies.
• Copper has an electron configuration that is an
exception to the aufbau principle.
• Cr, Cu, Nb, Mo, Ru, Rh, Pd, Ag, La, Ce, Gd, Pt, Au, Th, Pa,
• U, Np, Cm, Ds, Rg
•
• A. Half-filled and filled d subshells have unusual stability and this leads
to anomalies in electron configurations for some elements
• B. Most of these occur with atomic number (Z) > 40, where energy
differences between subshells are small. In all cases,
the transfer of an electron from one subshell (s) to another (d) lowers
the total energy of the atom because of a decrease in
electron- electron repulsion.
• Niobium Nb*41 [Kr] 4d45s1
• Molybdenum Mo* 42 [Kr] 4d55s1
• Ruthenium Ru*44 [Kr] 4d75s1
• Rhodium Rh* 45 [Kr] 4d85s1
• Palladium Pd* 46 [Kr] 4d105s0
• Silver Ag* 47 [Kr] 4d105s1
• Platinum Pt* 78 [Xe] 4f145d106s0
• Gold Au* 79 [Xe] 4f145d106s!
– A maximum of 2 electrons per orbital
Floating topic
Dimensional analysis/ factor method
The factor- method for solving numerical problems is a four-step
systematic approach to problem solving.
• Step 1: Write down the known or given quantity. Include both the numerical value and units of the quantity.
• Step 2: Leave some working space and set the known quantity equal to the
units of the unknown quantity.
• Step 3: Multiply the known quantity by one or more factors, such that the
units of the factor cancel the units of the known quantity and generate the
units of the unknown quantity.
• Step 4: After you generate the desired units of the unknown quantity, do
the necessary arithmetic to produce the final numerical answer.
Example: Convert 34 in. to cm 34 inx 2.54cm/1in= 86cm
common conversion factors
Length Volume Mass
1 m = 1.0936 yd 1 L = 1.0567 qt 1 kg = 2.2046 lb
1 in. = 2.54 cm (exact) 1 qt = 0.94635 L 1 lb = 453.59 g
1 km = 0.62137 mi 1 ft3 = 28.317 L 1 (avoirdupois) oz = 28.349 g
1 mi = 1609.3 m 1 tbsp = 14.787 mL 1 (troy) oz = 31.103 g
From defined quantities: The factors used in the
factor-unit method are factors derived from
fixed (defined) relationships between quantities.
• An example of a definition that provides factors is
the relationship between meters and centimeters:
1m = 100cm. This relationship yields two factors: 1m/100cm and 100cm/1m
A length of rope is measured to be 1834 cm. How many meters is this?
• Solution: Write down the known quantity (1834 cm). Set the known quantity equal to the units of the unknown quantity
(meters). Use the relationship between cm and m to write a factor (100 cm = 1 m), such that the units of the factor cancel the units of the known quantity (cm) and generate the units of
the unknown quantity (m). Do the arithmetic to produce the final numerical answer.
1834cm(1m/100cm)=18.34m
A length of rope is measured to be 1834 cm. How many
meters is this?
Solution: Write down the known quantity (1834 cm). Set the
known quantity equal to the units of the unknown quantity
(meters). Use the relationship between cm and m to write a
factor (100 cm = 1 m), such that the units of the factor cancel
the units of the known quantity (cm) and generate the units of
the unknown quantity (m). Do the arithmetic to produce the
final numerical answer
1834cm x1m/100cm = 18.34m
Measurements
Mole
Density
SI units
length (m) meter
kilometer (km) 1,000 m or 10^3 m
meter (m) 1 m or 100 m
decimeter (dm) 0.1 m or 10^-1 m
centimeter( cm) 0.01 m or 10^-2 m
millimeter (mm) 0.001 m or 10^-3 m
micrometer (mm) 0.000001 m or 10^-6 m
nanometer (nm) 0.000000001 m or 10^-9 m
amount of supstance (mol) mole
temperature (K) kelvin
The SI unit of temperature is the kelvin (K).
• No degree word nor symbol (°) is used with kelvin.
• The degree Celsius (°C) is also allowed in the SI system.
• Celsius degrees are the same magnitude as those of
kelvin, but the two scales place their zeros in different
places.
• Water freezes at 273.15 K (0 °C) and boils at 373.15 K (100°C).
temperature conversion
fahrenhight to celcicius
c=9/5(f-32)
Celsius to Fahrenheit
f=9/5(c)+32
kelvin to Celsius
C=k-273
Celsius
k=c+273
si unit prefexis
femto f 10^-15
pico p 10^-12
nano n 10^-9
micro m 10^-6
milli m 10^-3
centi c 10^-2
deci d 10^-1 kilo k 10^3
mega M 10^6
giga G 10^9
tera T 10^12
luminous intensity
candela (cd)
si us conversion factors
Length
2.54 cm = 1 in.
1 m = 39.4 in.
volume 946 mL = 1 qt
1 L = 1.06 qt
mass 454 g = 1 lb
1 kg = 2.20 lb
derived si unit
volume
definition
The measure of the amount of space occupied
by an object
The standard SI unit for volume
is the cubic meter
(m3), which is derived from the SI base unit of length
Other units for volume are the liter (L) and milliliter (mL)
1 dm3 = 1 L
• 1 cm3 = 1 mL
density
Density is a characteristic property of substances and
can be used to help identify substances.
Commonly used density units based on state of matter: g/ml
g/cm3 (solids, liquids)
g/L (gases)
mass/volume
MASS is the amount of matter an object contains
– Mass does not change unless you add or
remove matter
grams (g)
m=Dxv
A substance’s density is known to be 5.6g/mL. You have a
25.0mL sample of the substance. What is the mass of the
sample?
Solution: D = Mass/Volume
Density = 5.6 g/mL (given)
Volume = 25.0 mL (given)
Mass = ? (we have to solve for)
So we need to switch the density formula
Mass = D x V = 5.6 g/mL x 25.0 mL = 140 g (2 sig figs)
VOLUME is the amount of space an object
occupies
dicplacement method
The volume of irregularly
shaped objects may be
found by water
displacement
• measure a given amount
of water in a graduated
cylinder
• add the object and read
the volume of the water +
object
• then find the volume of the
object by subtraction.
Amount of H 2O with object = amount of H 2O without object =
Difference = Volume = ______
V=l x w x h.
A substance has a density of 13.5g/mL. You
have a 30.0g sample. What is the volume of your sample?
Solution: Density = Mass/Volume
Density = 13.5 g/mL (given)
Mass = 30.0 g (given)
Volume = ? (we have to solve for)
So we need to switch the density formula
Volume = Mass/Density = 30.0g/ 13.5g/ml = 2.22 mL (3 sig figs)
1mL = 1cm3
v=m/D
standerd si unit
The standard SI unit for density is the kilogram per cubic meter
(kg/m3)
The density of a substance is the ratio of the mass of a
sample of the substance to its volume.
The compactness and size of the molecules or particles
of a substance
– the more compact or squished together the molecules are and
the more mass the particles have, the larger the density
10.0mL sample of a sugar solution has a mass of
5.0 g. What is the density of the sugar solution?
Solution: D = Mass/Volume
Mass = 5.0 g (given)
Volume = 10.0 mL (given)
D = 5.0 g / 10.0 mL = 0.50 g/mL (2 s
time (s) Seconds
mass (kg) kilogram
kilogram(kg)
A kilogram was originally defined as the mass of a
liter of water.
• It is now defined by a certain cylinder of platinum-
iridium alloy, which is kept in France
significant figures
sig figs in a measurment include the known digits plus a final estimated dig. sig figs indicate percision of a meassurment
count all numbers exept
trailing zeros without a decimal point
examples
1. 23.50 4 sig figs ( trailing zeros are significant if there is a decimal)
2. 402 3 sig figs (zero is between non zero digits so it is significant
3. 5,280 3 sig figs ( trailing zeros with no decimal are not significant)
4. 0.080 2 sig figs ( first zero is not significant but 0 after 8 is
leading zeros
4. 0.080 2 sig figs ( first zero is not significant because it is a leading zero but the 0 after 8 is significant
Calculating with Sig Figs
Multiply/Divide
The # with the fewest sig figs
determines the # of sig figs in the answer.
(13.91g/cm3)(23.3cm3) = 324.103g
answer =324 g with (3sf)
4 SF - (13.91) 3 sf - (23.3) 3 SF= fewest sf so the answer should have 3 sf
15.30 g) ÷ (6.4 mL)
(4sf) ( 2 sf) (2sf)
= 2.390625 g/m 2.4g/ml
Add/Subtract -
The # with the lowest decimal value
determines the place of the last sig fig in the answer.
examples
18.9 g - 0.84 g
=18.06g anser =18.1g
ex. 1 3.75 mL
+ 4.1 mL
= 7.85 mL answer= 7.9ml the number with the least amount of sig figs is 4.1 and has 2 sf so the answer 7.9ml has 2 sf the calculater answer is 7.85 the las number is 5 so we round up to only 2sf and get 7.9 ml ex. 2 224 g
+ 130 g
= 354 answer= 350g 3 sf because the number with the lowest decimal value has 3 sf and the calculation showed 35(4) less than 5 so we round down to 0 so the answer is (350 g)
Count all numbers EXCEPT:
• Leading zeros -- 0.0025
• Trailing zeros without
a decimal point -- 2,500
Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
exact number
Exact Numbers do not limit the # of sig figs in the
answer
Average Atomic Mass Formula
example
* Counting numbers: 12 students
• Exact conversions: 1 m = 100 cm
• “1” in any conversion: 1 in = 2.54 cm
Atoms
Rutherford – planetary mode
Subtopic molecules
neutrons
Uncharged particle.
• Found within an atomic nucleus.
Isotops
The neutron with a charge of 0, a mass of 1.678 x 10-24 g, and located in the nucleus The neutron is slightly larger than the proton
summary
• Protons are located in the nucleus of
• an atom. They carry a +1 electrical charge and have a mass of 1
atomic mass unit (u).
• Neutrons are located in the nucleus of an atom. They carry no
electrical charge and have a mass of 1 atomic mass unit (u).
• Electrons are located outside the nucleus of an atom. They carry a -
1 electrical charge and have a mass of 1/1836 atomic mass unit (u).
They move rapidly around the heavy nucleus.
protons
Positively charged particle.
• Found within an atomic nucleus.
atomic number
Atomic number = the number of protons = the number of
electrons (if neutral)
electrons
The electron with a standardized charge of –1, a mass of 9.11 x 10-28
g, and located outside the nucleus in what is called the electron
cloud
The electron is ~ 1 / 1837 the mass of the proton
Negatively charged particle.
• Located in shells that surround an atom's
nucleus.
electron configuration
the atom contained a small, positively charged, dense
core or center called the nucleus
- the electrons traveled around outside the nucleus
- most of the atom’s volume was actually empty space
- the nuclear diameter is about 10-4 the diameter of the
atom
• Criticisms of the planetary model of the atom
• Why weren’t the electrons pulled into the positively charged nucleus
of the atom?
– Rutherford responded by stating the electron’s motion prevents it
from being pulled into the nucleus much the same as the planets
aren’t pulled into the sun or the moon into the earth
• According to classical mechanics (physics) charged particles
moving in a curved path should emit energy (light) or some other
form of electromagnetic radiation. Eventually, they would lose
enough energy to be pulled into the nucleus
Atoms are the particles that
make up molecules.
The Mole Concept Applied To Compounds
– The number of molecules in one mole of any compound is called
Avogadro's number and is numerically equal to 6.022x1023.
– A one-mole sample of any compound will contain the same
number of molecules as a one-mole sample of any other
compound.
– One mole of any compound is a sample of the compound with a
mass in grams equal to the molecular weight of the compound.
Examples Of The Mole Concept
– 1 mole Na = 22.99 g Na = 6.022x10^23 Na atoms
– 1 mole Ca = 40.08 g Ca = 6.022x10^23 Ca atoms
– 1 mole S = 32.07 g S = 6.022x10^23 S atoms
Calculate the number of moles of Ca contained in a 15.84 g sample of Ca
15.84 x 1 mole Ca/ 40.08g Ca answer 0.3952 moles Ca
Examples Of The Mole Concept
– 1 mole H2O = 18.02 g H2O = 6.022x1023 H2O molecules
– 1 mole CO2 = 44.01 g CO2 = 6.022x1023 CO2 molecules
– 1 mole NH3 = 17.03 g NH3 = 6.022x1023 NH3 molecules
The mole concept applied earlier to molecules can be applied to
the individual atoms that are contained in the molecules.
CO2
1 mole CO2 molecules = 1 mole C atoms + 2 moles O atoms
44.01 g CO2 = 12.01 g C + 32.00 g O
6.022x10^23 CO2 molecules = 6.022x10^23 C atoms +
(2) 6.022x10^23 O atoms
• Any two of these nine quantities can be used to provide factors for use in solving numerical problems.
Example 1: How many moles of O atoms are contained
in 11.57 g of CO2?
• Note that the factor used was obtained from two of the nine quantities given on the previous slide. 11.57gCO2 X 2 moles O atoms/ 44.01gCO2 = 0.5258 moles O atoms
Example 2: How many CO2 molecules are needed to
contain 50.00 g of C?
• Note that the factor used was obtained from two of the
nine quantities given on a previous slide.
50.00gCx6.022x10^23CO2 molecules / 12.01g C = 2.50707743...^24 answer = 2.507 x 10^24 CO2 molecules
Example 3: What is the mass percentage of C in CO2?
% C= mass of c/ mass of CO2 X 100
If a sample consisting of 1 mole of CO2 is used, the mole-based
relationships given earlier show that:
1 mole CO2 = 44.01 g CO2 = 12.01 g C + 32.00 g O
12.01g C/44.01g CO2 X 100=27.29% C
Example 4 What is the mass percentage of oxygen in CO2?
%O = mass of O / mass of CO2 X 100 the mass percentage is calculated using the following equation:
• Once again, a sample consisting of 1 mole of CO2 is used to take advantage of the mole-based relationships given earlier
where:
1 mole CO2 = 44.01g CO2 = 12.01 g C + 32.00g O
%o = 32.00 g O/44.01g CO2 X 100= 72.71%
neutron
Eventually, the neutron was discovered in 1932 when
James Chadwick used scattering data to calculate the
mass of this neutral particle.
– Chadwick is credited with his discovery
In 1928, a German physicist, Walter Bothe, and his student, Herbert Becker, took the initial step in the
search for the neutron. They bombarded beryllium with alpha particles emitted from polonium and found that it
gave off a penetrating, electrically neutral radiation, which they interpreted to be high-energy gamma
photons.
The discontinuous theory of matter – there existed
some smallet piece of matter
some smallet piece of matter
The discontinuous theory of matter – there existed
Continuous theory of matter – matter could be divided
forever without reaching a single smallest
indivisible unit
plum pudding model
Thomson – the plum pudding model
• a “sea” of positive charge with electrons scattered
throughout
• a divisible atom – subatomic particles existed
• an electrical nature associated with the atom
discovery of nucleus
The central part of an atom.
• Composed of protons and neutrons.
• Contains most of an atom's mass
Rutherford (1871 – 1973) along with coworkers Geiger,
Marsden, and Bohr devised and performed the gold foil
experiment in 1911 The experiment: A thin sheet of gold foil was enclosed by a fluor covered circular shroud
– There was an opening in the shroud through which
alpha particles were shot at the gold foil
Most of the alpha particles passed through the foilwithout deflection because the vast majority of the atom
is empty space with a few electrons in it
2) The few alpha particles deflected at minor angles came close to a small bundle or core of positive charge which were, therefore, repelled
3) The scant number of alpha particles being bounced back
was a result of an almost direct collision with a very massive (extremely dense) and positively charge region ccupying a very small volume
Atom Is Neutral
Atoms have no overall electrical charge so,
an atom must have as many electrons
as there are protons
in its nucleus. Atom -
• Nucleus: Proton + Neutron
• Electron
discovery of electron
J.J. Thomson deflects the cathode ray with an electrical field
– The rays bend toward the positive pole, therefore, they are negative
– 1897 announces that the corpuscles (electron) is a negatively charged particle
as it is deflected towards t
proton
Credit for the discovery of the proton is debatable pending
upon source
- However, some people credit Rutherford with its discovery the proton’s mass was eventually identified as 1.672 65 x
10-24 g
scientific notation
a x 10 ^n
a = # 1-10 is a number greater than or equal to 1 but less than 10
Subtopic
n= whole number # Grater than 1 have a positive exponent numbers less than 1 have a negative exponent
Positive exponent ex. 2.35x 10^8 Negative exponent ex. 3.97 x 10^-9
Rules for Division
When dividing numbers in scientific notation, divide the first
factor in the numerator by the first factor in the denominator.
Then subtract the exponent in the denominator from the
exponent in the numerator.
Ex Divide 6.4 x 10^6 by 1.7 x 10^2
(6.4) (1.7) = 3.76
(6) - (2) = 4 or 10^4
3.76 x 104
Ex Divide 2.4 x 10^-7 by 3.1 x 10^14 answer 7.74 x 10^-22
..
Rule for Addition and Subtraction
In order to add or subtract numbers written in scientific notation, you must express them with the same power of 10.
Sample Problem: Add 5.8 x 103 and 2.16 x 104
(5.8 x 10^3) + (21.6 x 10^3) = 27.4 x 103
Exercise: Add 8.32 x 10^-7 and 1.2 x 10^-5 1.28 x 10-5
2.7^4 x 10^4
rules for multiplication
When multiplying numbers in scientific notation, multiply the
first factors and add the exponents.
Multiply 3.2 x 10^-7 by 2.1 x 10^5
(3.2) x (2.1) = 6.72
add exponents (-7) + (5) = -2 or 10^-2 Answer = 6.72 x 10^-2
Exercise: Multiply 14.6 x 107 by 1.5 x 104 answer 2.19 x 10^12
scientific notation to standard notation
When changing scientific notation to standard notation, the exponent tells you which way to move the decimal and how many spaces *If an exponent is positive, the number gets larger, so move the decimal to the right.
• If an exponent is negative, the number gets
smaller, so move the decimal to the left.
The exponent tells how many spaces to
move the decimal:
4.08 x 10^-3 = 4 0 8
In this problem, the exponent is -3, so the
decimal moves 3 spaces to the left.
The exponent also tells how many spaces to
move the decimal:
4.08 x 10^3 = 4 0 8
In this problem, the exponent is +3, so the
decimal moves 3 spaces to the right.
positive exponent go right
With a positive exponent, move the decimal to
the right:
4.08 x 10^3 = 4 0 8 0
Don’t forget to fill in your zeroes!
negative exponent go left
With a negative exponent, move the decimal to
the left:
4.08 x 10^-3 = 000.4 0 8
Don’t forget to fill in your zeroes!
examples Try changing these numbers from Scientific
Notation to Standard Notation:
1) 9.678 x 10^4
2) 7.4521 x 10^-3
3) 8.513904567 x 10^7
4) 4.09748 x 10^-5 answers
96780
.0074521
85139045.67
.0000409748
scientific notation is used to express very small or very large numbers and maintain correct number of significant figures
Express use scientific calc (EXP) botton 1.8 x 10^-4 in decimal notation.
anser 0.00018
Express 4.58 x 10^6 in decimal notation.
4,580,000
On the graphing calculator, scientific notation is done with
the button. (EXP)
4.58 x 10^6 is typed 4.58x10 (EXP) 6
standerd notation to scientific notation
original number is greater than 1; the exponent will be positive 348943 = 3.489x10^5
original number is less than 1 the exponent will be negative ex scientific notion of .000000672 = 6.72x10^-8
1) First, move the decimal after the first non zero digit
Ex. 3 2 5 8
count 123 decimal moved 3 spaces
2) Second, add your multiplication sign and your base (10).
3 . 2 5 8 x 10
3) Count how many spaces the decimal moved and this is the exponent.
3 . 2 5 8 x 10^3
Express 0.0000000902 in scientific notation.
Where would the decimal go to make the number be between 1 and 10?
9.02
The decimal was moved how many places?
8
When the original number is less than 1, the exponent
is negative.
9.02 x 10^-8
Matter
weight is the gravitational force acting on an object and can differ depending on location
matter is anything that has mass and takes up space
mass is a measurement of the amount of matter an object has. mass of an object stays the same anywhere
isotopes
Atomic Mass on the Periodic table is the average mass of the isotopes
– But the mass number of each isotope is the protons plus the neutrons
Isotopes
of an element have
different mass numbers
because they have different numbers of neutrons, but they have
the same atomic number.
atomic mass
Atomic Mass Unit
is a unit used to compare
the masses of atoms
and has the symbol
u or amu.
1 amu or u is approximately equal to the mass of
a single proton or neutron.
Carbon-12
Chemists have defined
the carbon-12 atom
as having a mass of
12 atomic mass units.
1 u = 1/12 the mass of a Carbon-12 atom.
1 u = 1/12 the mass of a Carbon-12 atom.
12 atomic mass units.
the carbon-12 atom
• Isotopes are atoms that have the same number of
protons in the nucleus but different numbers of neutrons.
That is, they have the same atomic number but different
mass numbers.
• Because they have the same number of protons in the
nucleus, all isotopes of the same element have the
same number of electrons outside the nucleus.
Isotopes of Carbon and Hydrogen
Isotopes of Hydrogen
protium deuterium tritium
11H 21H 31 H
Isotopes of Carbon
116C 12 6C 136C 146C 156C 166 C
3617Cl OR Cl-36
Isotope Symbols
• Isotopes are represented by the symbol AzE
• where Z is the atomic number, A is the mass number, and is the
elemental symbol.
3 phases/ states of mater
plasma
A plasma is an ionized
gas.
A plasma is a very good
conductor of electricity
and is affected by
magnetic fields.
Plasmas, like gases
have an indefinite shape
and an indefinite volume. • Plasma is the
fourth state
of matter
Plasma is found in certain high temperature
environments.
• Naturally: Stars, lightning.
• Man-made: Television screens.
19
gas- have an
indefinite shape and
an indefinite volume. both shape and volume of Particles of gases are
very far apart and
move freely. expands to fill container
solid- rigid and has fixed shape and volume
liquid- flows and takes (indefinite) shape of its container and has fixed (defintie)volume
Dalton’s Atomic Theory
-All elements are made of tiny atoms.
– Atoms cannot be subdivided.
– Atoms of the same element are exactly alike.
– Atoms of different elements can join to form
molecules
According to John Dalton, matter is made of very tiny particles called atoms. And atoms cannot be broken down
further.
• That is atoms were not supposed to be composed of simpler constituents.
They were imagined to be like marbles
Kinetic Theory of Matte
Ave. At. Mass = [(% x isotope mass) + (% x isotope mass) + .....]/ total %
sotopes And Atomic Weight Example
A specific example of the use of the equation is shown below for the element boron that consists of 19.78% boron-10 with
a mass of 10.01 u and 80.22% boron-11 with a mass of 11.01u. this calculated value is seen to agree with the value given in the periodic table.
AW(average weight)= (19.78%)10.01u)+(80.22%)(11.01u) / 100/= 198.u+883.2u/100= 10.81u
Particulate Theory of Matter
All matter is made up of tiny
particles called molecules and
atoms.
• Molecules
A molecule is the smallest
particle of a pure substance that
is capable of a stable
independent existence.
Atoms
• Atoms are the particles that
make up molecules.
Particulate Theory of Matter
The kinetic molecular theory of matter is a useful tool
for explaining the observed properties of matter in the
three different states of solid, liquid and gas.
– Postulate 1: Matter is made up of tiny particles called
molecules.
– Postulate 2: The particles of matter are in constant
motion and therefore possess kinetic energy.
– Postulate 3: The particles possess potential energy
as a result of repelling or attracting each other.
– Postulate 4: The average particle speed increases as
the temperature increases.
– Postulate 5: The particles transfer energy from one to
another during collisions in which no net energy is lost
from the system.
Law of conservation of matter
There is no detectable change in the total quantity of matter present when matter converts from one type to
another.
• This is true for both chemical and physical
changes.
classification of matter
Mixtures
Mixtures can vary in composition and properties.
Example: mixture of table sugar and water (can have different proportions of sugar and water)
• A glass of water could contain one, two, three, etc. spoons of sugar.
• Properties such as
sweetness would be
different for the mixtures
with different proportions.
two types of mixtures
A heterogeneous mixture has a composition that
varies from point to point.
Oil and vinegar salad dressing is a heterogeneous mixture because its composition is not uniform
throughout A pizza pie is a heterogeneous mixture.
A piece of crust has different properties
than a piece of pepperoni taken from the
same pie.
A homogenous mixture exhibits a uniform composition
and appears visually the same throughout. Another name for a homogenous mixture is a solution.
A commercial sports drink is a homogeneous mixture because its composition is uniform throughout.
Homogeneous mixtures are also called
solutions. The properties of a sample of a
homogeneous mixture are the same regardless
of where the sample was obtained from the
mixture
pure substances
Compound
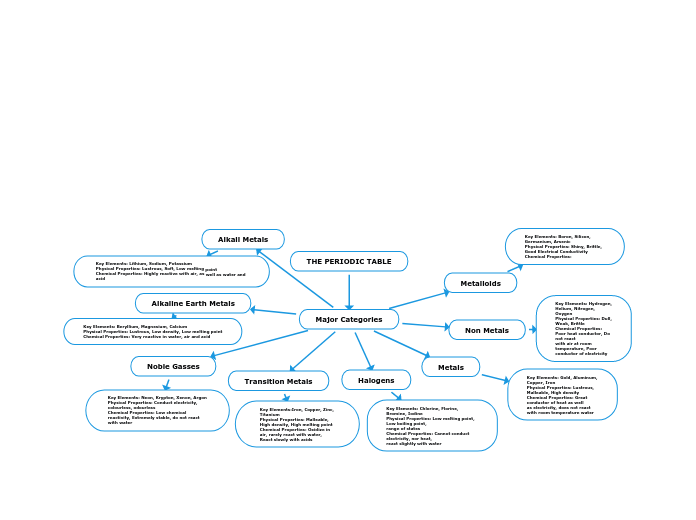
Periodic table
The periodic table is useful in predicting:
1. Chemical behavior of the elementsChemical behavior of the elements
2.2. TrendsTrends
3.3. Properties of the elementsProperties of the eleme
Three Types of Elements
• Nonmetals
Upper Right side of the Periodic Table
Generally brittle solids or solids or gases
Poor conductors of heat and electricity
Bromine is the only liquid at room temperater
• Metalloids
A.k.a – the semi-metals
Boxes bordering the stair-step
Physical and chemical characteristics of both metals and non metal
• Metals
Shiny when smooth and clean
Solid at room temperatur
• Only exception - Mercury
Good conductors of heat and electricity
Most are ductile and malleable
history
John Newlands
Noticed when elements wereNoticed when elements were
arranged byarranged by atomic massatomic mass,,
they repeated propertiesthey repeated properties
everyevery 88thth element.element.
He used the wordHe used the word periodicperiodic toto
describe this patterndescribe this pattern
He gave it the name theHe gave it the name the LawLaw
ofof OctavesOctaves1838-1898
Did not work for all the elementswork for all the elements
Criticized because of its association withCriticized because of its association with musicmusic
Did give others the idea of repeating properties -Did give others the idea of repeating properties -
periodicperiodic
We Hate It
Lothar MeyerMeyer and Dmitriand Dmitri MendeleevMendeleev
Each made a connection betweenEach made a connection between atomic massatomic mass and and propertiesproperties of elementsof elements
1830-1895 1834 - 1907
Mendeleev is given credit because his was
publishedpublished firstfirst
In addition, Mendeleev predictedIn addition, Mendeleev predicted unknownunknown
elementselements
However, not completely correct –However, not completely correct – newnew elementselements
werenweren’’t int in correctcorrect orderorder
What do you notice about elements 27 & 28 andWhat do you notice about elements 27 & 28 and
52 & 53?52 & 53?
MendeleevMendeleev
Henry Moseley
Solved this problem bySolved this problem by
arranging the elements byarranging the elements by
increasingincreasing atomic number.atomic number.
TheThe periodicperiodic repetition ofrepetition of
chemical and physicalchemical and physical
properties of elementsproperties of elements
when arranged by atomicwhen arranged by atomic
numbernumber is now known asis now known as
PeriodicPeriodic LawLaw
1887-1915
Which leads to the Modern Periodic Table
Boxes each with:Boxes each with:
H
1
Hydrogen
1.00794
Element Name
Atomic Number
Atomic Symbol
Atomic Mass
That are arranged by increasing atomic numbers
Atomic number = the number ofAtomic number = the number of protonsprotons = the= the
number ofnumber of electronselectrons (if neutral)(if neutral)
Atomic Mass on the Periodic table is the
average mass of the average mass of the isotopes-
• But the mass number of each isotope is the is the protons plus the neutrons
∆U ≡ ionization energy, IE
decreasing size, increasing IE
The minimum energy required to remove an e from the ground state of a gaseous atom
• II11 - the energy needed to remove the- the energy needed to remove the first electron from a gaseous atom.
K(g) +]+ I1 K+(g) + e–– E = +419 kJ/mol
• II22 - the energy needed to remove the second-
electron from a gaseous +1 ion.
• K+(g) +I2 K2+(g) + e = +3051 kJ/mol
decreasing size, increasing iE
Summary of Periodic Trends
moving through the periodic table: atomic radius ionization energy electron affinity down group increase decrease less exothermic across a period decrease increase more exothermic
columns
The columns are called FamiliesFamilies oror Groups
• Earlier Version had 1-8 followed by A or b
Group A elements are called Representative Elements
Group B elements are called TransitionTransition ElementsElements
• Modern Version labels the columns with 1-18Modern Version labels the columns with 1-18
element symbol
Chemical Symbol
The symbol that refers to theThe symbol that refers to the elementelement
First letter isFirst letter is capitalizedcapitalized, second letter (if, second letter (if
applicable) isapplicable) is lowerlower casecase
Not all symbols are based on English names forNot all symbols are based on English names for
the elements, some come from their Latinthe elements, some come from their Latin
names or even other languagesnames or even other languages
– Silver – Ag – argentum
– Antimony – Sb -stibium
– Lead – Pb – plumbum
– Copper – Cu – cyprium
– Tin – Sn – stannum
– Iron – Fe - ferrum
– Mercury – Hg - hydrargyrum
– Gold – Au - aurum
molecules
Homoatomic Molecules
• The atoms contained in homoatomic molecules areof the same kind.
Heteroatomic Molecules
• The atoms contained in heteroatomic molecules are of two or more kinds.
Classify the molecules using the terms homoatomic or heteroatomic molecules.
• Solution: H2O2 and H2O are heteroatomic molecules and O2
is a homoatomic molecule.
Molecules
• Only a few elements exist as individual atoms.
• Most elements exist as molecules where two or more
atoms of the same element are bonded together.
The elements hydrogen, oxygen, phosphorus, and sulfur form molecules consisting of two or
more atoms of the same element.
* Diatomic molecules contain two atoms.
• Triatomic molecules contain three atoms.
• Polyatomic molecules contain more than three atoms.
Classify the molecules in these diagrams using the terms
diatomic, triatomic, or polyatomic molecules.
• Solution: H2O2 is a polyatomic molecule, H2O is a triatomic molecule, and O2 is a diatomic molecule
A molecule is the smallest
particle of a pure substance that
is capable of a stable
independent existence
A symbol is assigned to each element. The symbol is based on
the name of the element and consists of one capital letter or a
capital letter followed by a lower case letter.
– Some symbols are based on the Latin or German name of the
element.
Select the correct symbol for each:
A. Calcium
1) C 2) Ca 3) CA
B. Sulfur
1) S 2) Sl 3) Su
C. Iron
1) Ir 2) FE 3) Fe
A. Calcium
2) Ca
B. Sulfur
1) S
C. Iron
3) Fe
Select the correct name for each:
A. N
1) neon 2) nitrogen 3) nickel
B. P
1) potassium 2) phogiston
3) phosphorus
C. Ag
1) silver 2) agean 3) gold
Select the correct name for each:
A. N
2) nitrogen
B. P
3) phosphorus
C. Ag
1) silver
Element is the simplest form of matter that
can not be broken down by chemical means;
One element can be changed into another
element by nuclear methods
• Are the building blocks of matter
• Currently: Type of matter composed of atoms that all have the same atomic #s (Identical atoms)
• 118 elements known today Of the 118 known only the first 98 are known to
occur naturally on earth
• Those that do not occur naturally have been artificially produced by man as synthetic products of nuclear reactions such as Einsteinium ,
Nobelium
O 65.0 % K 0.34
C 18.5 S 0.26
H 10.0 Na 0.14
N 3.0 Cl 0.14
Ca 1.4 Fe 0.004
P 1.0 Zn 0.003
Mg 0.50
Trace Elements:
As, Cr, Co, Cu, F, I, Mn, Mo, Ni, Se, Si, V
Hydrogen (H) The lightest & the most abundant element in the universe [75%, followed by Helium 23%]
• Carbon (C) The 2 nd most abundant
element[18.5%] in human body after
Oxygen
• Oxygen (O) The most abundant element on earth crust [47% followed by Si 28%]
• A substance composed of a
single kind of atom.
• Cannot be broken down into
another substance by chemical
or physical means.
Formulas are used to represent compounds.
A compound formula consists of the symbols of the
elements found in the compound. Each elemental
symbol represents one atom of the element. If more
than one atom is represented, a subscript following the
elemental symbol is used.
• Carbon
monoxide, CO
– one atom of C
– one atom of O
• Water, H2 O
– two atoms of H
– one atom of O
• Ammonia, NH 3
– one atom of N
– 3 atoms of H
if carbon disulfide contains one atom
of carbon for every two atoms of sulfur, what is the chemical formula for carbon disulfide?
CS2
rows
periods
Rows are called Periods
• Seven Seven periods for the seven energy levels(rings)
Compounds are pure substances that are made up of heteroatomic molecules or individual atoms (ions) of two or more different kinds
Examples: pure water made up of heteroatomic
molecules and table salt made up of sodium atoms (ions) and chlorine atoms (ions)
Compounds – Pure substances that CAN be broken
down into simpler substances by chemical changes.
• Consist of two or more types of elements chemically
bonded • The properties of compounds are different from the
uncombined elements making up the compound.
Pure substances and mixtures
Examples: H2O, C6H12O6, AgCl
elements
- The number of atoms in one mole of any element is called Avogadro's number and is equal to 6.022x10^23 .
– A one-mole sample of any element will contain the same
number of atoms as a one-mole sample of any other element.
– One mole of any element is a sample of the element with a
mass in grams that is numerically equal to the atomic weight of
the element
Elements are pure substances that are made up of homoatomic molecules or individual atoms of the same kind
Examples: oxygen gas made up of homoatomic
molecules and copper metal made up of individual
copper atoms
Pure substances have constant composition.
– Elements – Pure substance that CANNOT be broken
down into simpler substances by chemical changes.
• Consist of one type of element.
Examples: Gold (Au), Phosphorus (P4), Oxygen (O2)
example
Matter Classification Example
• Classify H2, F2, and HF using the classification scheme from
the previous slide.
• Answer:
– H2, F2, and HF are all pure substances because they have a constant composition and a fixed set of physical and
chemical properties.
– H2 and F2 are elements because they are pure substances composed of homoatomic molecules.
– HF is a compound because it is a pure substance
atomic weight/ average atomic mass
Atomic Weight (Average Atomic Mass)
is the weighted average
mass of all the naturally occurring
isotopesof that element.
Average Atomic Mass
• The average atomic mass may be determined by
calculation from any analysis yielding the actual masses
of the isotopes and some relative proportion of their
presence (an actual number of nuclides or aspercentages of nuclides)
• A “rough estimate” may be obtained if the mass numbers
are utilized
Average Atomic Mass
In looking at the masses of the elements on the periodic
table it is evident that most values listed aren’t close to
being whole numbers
Why?
These values are actually weighted averages that represent
all the naturally occurring isotopes and the relative
abundance in which they are found in nature
The proton with a stadardized charge of +1, a mass 1.673 x 10-24 g,
and located in the nucleus
properties
intensive property
– Does not depend on the amount of matter
present.
– Examples: density, temperature
Extensive property
Depends on the amount of matter present
Examples: mass, volume, heat
The characteristics that enable us to distinguish one substance from another are called properties
Phisical property
A physical property is a characteristic of matter that is not associated with a change in its chemical composition Physical properties can be observed or
measured without attempting to change the
composition of the matter being observed
Examples: density, color, hardness, melting and boiling points, and electrical conductivity.color, shape and mass
Subtopic
chemical properties
Chemical properties can be observed or
measured only by attempting to change the
matter into new substances.
Examples: flammability and the ability to react
(e.g. when vinegar and baking soda are mixed) One of the chemical properties of iron is that it rusts; (b) one of the chemical properties
of chromium is that it does not.