Floating topic
Energy Changes
Chemical Reactions
Endergonic
accompanied by or requiring the absorption of energy, the products being of greater free energy than the reactants.
Energy required, nonspontaneous
products have more energy than the reactants
If the products have more energy than the reactants, then you can see that energy was absorbed through the process of the reaction
Delta G is positive
Exergonic
An exergonic reaction (such as cellular respiration) is a reaction that releases free energy in the process of the reaction.
energy released, spontaneous
The Overall delta G is negative
if the reaction proceeds with a net release of energy then the products have lower free energy than what the reactants started
Free energy
Energy in cells that is used for a cell to function
Non-Spontaneous Reaction
a reaction that does not favor the formation of products at the given set of conditions. In order for a reaction to be nonspontaneous, it must be endothermic,
Spontaneous Reaction
Only reactions with a negative ΔG are spontaneous, which means they occur with no net input of energy.
Spontaneous reactions can be harnessed to perform cellular work
Thermodynamics
The study of energy transformations is called thermodynamics. Scientists use the terms system and surroundings in this field. System is the matter under study, while the rest of the universe is the surrounding
2nd Law
Every energy transfer or transformation increases the entropy of the universe
1st Law
Energy can be transferred and transformed, but it cannot be created or destroyed
plant cells
hypotonic - turgid/ normal (plants need a lot of water to survive)
hypertonic - plasmolyzed (loss of water/ cell can die)
isotonic - flaccid (not net movement of water and can cause plant to wilt)
animal cells
hypotonic - lysed
isotonic - normal
hypertonic - shriveled
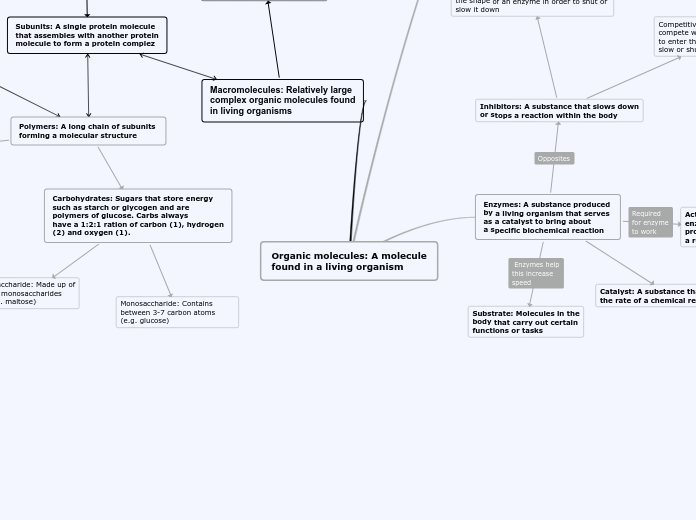
Metabolism
Metabolic pathway
Anabolic Pathway
Anabolic and catabolic pathways: Anabolic pathways are those that require energy to synthesize larger molecules.
Pathways that consume energy to build larger, complicated molecules from simpler ones
Photosynthesis 6CO2 +6H20 + light-> C6H1206 + 6O2
Example is photosynthesis – where energy from the sun is used to combine carbon dioxide and water to form sugars.
Polymerization
Polymerization is a process through which a large number of monomer molecules react together to form a polymer.
Biosynthetic pathways
There are two known pathways for the biosynthesis of glycine.
Catbolic Pathway
Metabolism manages the material and energy resources of the cell
Pathways that release energy by breaking down complex molecules into simpler compounds
Celular resperation
Cellular respiration is a series of chemical reactions that break down glucose to produce ATP, which may be used as energy to power many reactions throughout the body.
C6H12O6+6O2->H2O + Energy
a complex molecule like glucose is broken down to carbon dioxide and water with the release of energy.
2. depolarization
Depolarization occurs when ion channels cause the inside of the cell to become less negative. It is a reduction in the magnitude of the membrane potential. This also triggers an action potential.
generation of action potential
4. falling phase/ repolarization
The inside of the cell becomes negative again.
3. rising phase
The voltage begins to increase until it reaches action potential.
1. resting state
Most voltage gated Na+ and K+ channels are closed.
5. undershoot
The K+ channels have to close up again and before this happens there is a slight overly negative charge in the cell before it is able to reach its resting state once again.
these pumps help maintain membrane potential
Energy transfer and
transformation is critical to
all aspects of biology from
cells to ecosystems
Forms of Energy
Potential energy
An object that is not moving can also have energy – potential. This is due to location or structure.
Stored Energy
Happens Because of position, location, or arrangement
Electron in outer shell - more potential energy
Potential energy in foods is chemical energy
chemical energy stored in molecular structure
Kinetic energy
If energy is associated with the relative movement of objects it is called kinetic energy. An example is thermal energy – kinetic energy associated with random movement of atoms of molecules Light is a form of kinetic energy in the form of photons moving from one place to another.
Light energy
Kinetic energy of movement of photons
Thermal energy
Kinetic energy of molecular motion
signal molecule/ligand and a receptor
How does a signal work using cAMP?
Messenger binds to GPCR to activate it
|
Activated GPCR binds to G protein then binds to GTP which activates G protein
|
Activated G protein/GTP binds to adenylyl cyclase, GTP is hydrolyzed which activates adenylyl cyclase
|
Activated adenylyl cyclase converts ATP to cAMP
|
cAMP, a second messenger activates another protein leading to a cellular response
Stage 1: Reception
Stage 2: Transduction
Stage 3: Response
Signal Transduction
Cells communicate using rather physical contact or releasing a signal to a target cell
use
Active transport
Needed protein to transfer molecules
Exocytosis
Some inside cells are gotten rid out membrane
Endocytosis
eat outside cells through membrane
Pumps
Protien=ATD
Contractile Vacuole
NA+/K+ pump
Requires energy
From low concentration to high concentration
long distance signaling - if the cell is releasing the signal from a far distance to a receptor
local signaling - exchanging of signals within close proximity
paracrine or synaptic
Enzymes
enzymes lower the energy of activation barrier: exergonic reaction to change in the presence of enzyme
If a reaction is spontaneous/exergonic then it doesn’t require any energy input
Environmental Factors Affecting Enzyme Activity
pH
cell’s neutral pH is 7.2 and this is where most enzymes function, there are some exceptions as seen here.
Enzyme Regulation
Allosteric regulation
Regulatory molecule: Inhibitor or Activator
Regulator molecule binds to a protein at one site and affects the protein’s function at another site. Allosteric regulation may either inhibit or stimulate an enzyme’s activity
Allosteric Regulation
Allosteric regulation occurs when a regulatory molecule (inhibitor, activator) binds to a protein at one site and affects the protein’s function at another site. Allosteric regulation may either inhibit or stimulate an enzyme’s activity
Cooperativity
type of allosteric regulation. In cooperativity, the binding of one substrate molecule to the active site of one subunit locks all other subunits into the active shape. Cooperativity amplifies the response of enzymes to substrates
Inhibition of Enzyme Activity
Noncompetitive inhibition
A noncompetitive inhibitor binds to the enzyme away from the active site, altering the shape of the enzyme so that even though the substrate can still bind, the active site functions much less effectively, if at all.
Competitive inhibition
A competitive inhibitor mimics the substrate, competing for the active site.
Temperature
rate or speed of a reaction increases with increase in temperature
The ATP Cycle
ATP becomes ADP and the energy which is stored is released to help out some biological function. Later when a phosphate group is added, ADP is recharged back to ATP. This cyclic transformation from ATP to ADP and again back to ATP is called as ATP cycle.
ATP is a renewable resource that is regenerated by addition of a phosphate group to ADP. The energy to phosphorylate ADP comes from catabolic reactions in the cell.
Both endergonic and exergonic reactions occur in cells
couple these two by using coupling agents like ATP
ATP
ATP is called the energy coupler or the currency of the cell.
Mechanical work
ATP binds noncovalently to motor� proteins and then is hydrolyzed
Transport work
ATP phosphorylates transport proteins
Energy coupler in cells:cellular work
Chemical reactions powered by ATP
formation of glutamine (amino acid)
this reaction is coupled with ATP hydrolysis,
Pi formed is added to one of the reactants glutamic acid
makes it unstable (high free energy).
its attempt to be more stable reacts with ammonia to form glutamine and the Pi group is released in the process
Coupling is favored
Flow of Electrons
Cyclic
Anaerobic Conditions
Synthesis of ATP
Non-Cyclic
Aerobic Conditions
Produces NADPH in addition to ATP
Facilitates the synthesis of organi moelcules and extended storage of energy
Photosystems
Photosystem 2
The reaction - center chlorophyll 'a' absorbs at 680 nm
Photosystem 1
The reaction - center chlorophyll 'a' absorbs at 700 nm
Output: 6 NADH, 2 FADH, 2 ATP
Output: 2 Pyruvate, 2 NADH, 4 ATP
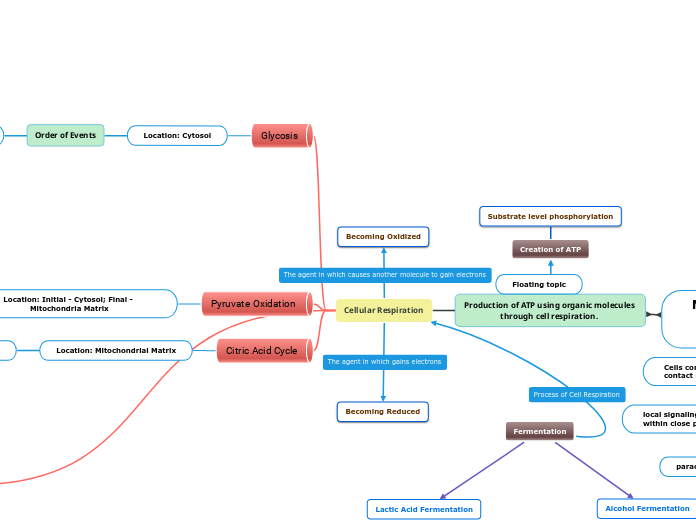
Creation of ATP
Substrate level phosphorylation
Becoming Reduced
Becoming Oxidized
Fermentation
Alcohol Fermentation
Converts sugars into cellular energy producing ethanol and carbon dioxide as by products.
Lactic Acid Fermentation
A process in which sugars are converted into cellular energy in which lactic acids is a part of the solution.
Gibbs Free energy
G > 0
A reaction/process cannot occur spontaneously. An input of free energy is required to drive the reaction. Reaction is endergonic.
G = 0
A system is at equilibrium: no net change occurs
G < 0
A reaction/process can occur spontaneously. Reaction is exergonic
Gfinalstate – G initialstate or Gproducts – G reactants
Membranes, Energy, and Cell Communication
Energy transfers and transformations in cells
Subtopic
Cell Signaling
components of membranes
membrane potential
Membrane potential is the amount of voltage in the cell. When there is no net movement of certain ions across the membrane then equilibrium potential is reached.
ion channels
Ion channels are membrane proteins that allow for transport of ions across membranes. They are always open but can be closed at times. This would depend on the voltages within the membrane.
gated
Ion channels are able to open and close in response to stimuli.
stretch gated - open when membrane is deformed
ligand gated - open and close when a neurotransmitter binds to channel
voltage gated - open and close in response to changes in membrane potential
ungated
Ion channels are always open and allow for ions to move through.
selective permeability
A cell has to regulate transport of substances across the cellular membrane. This is important because it helps manage and separate different materials traveling throughout the cell. Small nonpolar molecules and small uncharged polar molecules have higher permeability and can travel through the bilayer. Large uncharged polar molecules and ions have low permeability and cannot pass through the membrane on its own.
active transport
Active transport moves substances from low to high concentration, against concentration gradients, requiring energy (ATP).
bulk transport
Large molecules cross the membrane in bulk in vesicles.
endocytosis
Endocytosis takes in molecules. There are three types: phagocytosis, pinocytosis, receptor-mediated endocytosis
phagocytosis takes in large food particles/ other cells by extending its membrane out
pinocytosis takes in extracellular fluid from outside in vesicles
receptor mediated endocytosis is a specialized type of pinocytosis that allows for the cell to acquire bulk quantities of specific substances even if they may not be concentrated in the extracellular fluid
exocytosis
Transport vesicles move towards the membrane, fuse with it and release their contents. Exocytosis is used to export products.
cotransport
Cotransport occurs when active transport of a solute indirectly drives transport of other substances. For example, plant cells use the H+ generated from proton pumps to drive active transport of nutrients into the cell.
NA+/ K+ pump
The sodium potassium pump aids in the transport of sodium and potassium ions in a cell. There is usually in abundance of sodium outside the cell and abundance of potassium inside the cell. 3 Na+ are transported out of the cell for every 2 K+ ions transported in.
types of electrogenic pump
Electrogenic pumps are proteins that generate voltage across a membrane. These also help store energy used for cellular work.
proton pumps
A positive charge leaves the cell, and a slight negative charge develops inside the cell and a positive charge outside the membrane.
passive transport
Passive transport is a movement across a membrane that does not require any energy. It is moving down the concentration gradient, from an area of high concentration to low concentration.
osmosis
Osmosis is the diffusion of water across a selectively permeable membrane. Water diffuses from an area of lower solute concentration to an area of higher solute concentration until the concentrations are equal on both sides.
water balance in cells
tonicity
tonicity is the ability of a surrounding solution to cause a cell to gain or lose water
hypotonic
solute concentration is less than that inside the cell with respect to the cell/ solute concentration is less than that outside of the cell with respect to the environment
hypertonic
solute concentration is greater than inside the cell with respect to the cell/solute concentration is greater than that outside of the cell with respect to the environment
isotonic
solute concentration is the same as inside the cell; no net water movement
diffusion
Diffusion is the tendency for molecules to spread out evenly into available space. They always move from areas of high concentration to low concentration.
facilitated diffusion
Facilitated diffusion is passive transport aided by proteins. They help move hydrophilic substances across membranes as they cannot diffuse on their own.
carrier proteins
Carrier proteins undergo subtle changes that help transport proteins from on side to another. Sort of like an elevator, it takes in a few molecules at a time, closes, and opens up on the other side.
channel proteins
Channel proteins provide channels that allow a specific molecule or ion to cross the membrane.
aquaporin
Aquaporin are types of proteins that aid in the transport of water across membranes.
mosaic plasma membrane
two different types of proteins present in membranes
integral
Integral proteins are partially or fully inserted into the membrane. If they are fully inserted than they are called transmembrane proteins.
transmembrane proteins/ transport proteins
peripheral
Peripheral proteins are anchored to the membrane
phospholipid bilayer
membrane fluidity
presence of cholesterol in animal cells
the presence of cholesterol regulates the movement of phospholipids in membranes; at moderate temperatures it helps to reduce movement while in low temperatures, it prevents packing between phospholipids
types of fatty acids present
unsaturated fatty acids provide a bend in the structure of the phospholipid bilayer, allowing for more fluidity; the presence of unsaturated fatty acids also helps the membrane withstand low temperatures
temperature
each phospholipid has a specific phase transition temperature; above this temperature, the lipid is in liquid crystalline phase and fluid; below this temperature, the lipid is in a gel phase and is rigid
Production of ATP using organic molecules through cell respiration.
Cellular Respiration
Oxidative Phosphorylation
Location: Mitocondria
Chemiosmosis
Comparison
Chloroplast Strucutre
Thylakoid Space
Thylakoid Membrane
Stroma
Mitochondrion Structure
Intermembrane Space
Inner Membrane
Matrix
Transporting of Proton through channels in the membrane of mitochondria from the inner and outer compartments.
The constructing of a proton (H+) gradient
Protons diffuse down the gradient though a protein
Coupled to ATP Synthase
Electron Transport Chain
The electrons go through the chain higher to lower energy levels.
The energy released in the ETC is then used as a proton gradient,
Citric Acid Cycle
Location: Mitochondrial Matrix
Input: 2 Acetyl CoA
Malate is Oxidizde, NAD+ is reduced
Succinate is oxidized, FAD is reduced.
ATP formation
Once CO2 is released, the 4 carbon molecule is oxidized, then reactive due to additional CoA
Isocitrate is oxidized, NAD+ is reduced
The Acetyle CoA+Oxaloacetate from Citrate.
Pyruvate Oxidation
Location: Initial - Cytosol; Final - Mitochondria Matrix
Input: 2 Pyruvate
A carboxyl group is removed from pyruvate, NAH+ is reduced, then a acetyl groups is transferred to coenzyme A.
Output: 2 Acetyl CoA, 2 NADH
Glycosis
Location: Cytosol
Order of Events
Input: 1 Glucose, 2 ATP
Energy Payoff Phase
Step 10
The phosphate group is transferred from PEP to ADP forming pyruvate
Step 9
The enolase removes a water molecule from 2-phosphoglycerate, yielding phosphoenolpyruvate.
Step 8
The enzyme relocates the remaining phosphate group
Step 7
The phosphate group is transferred to ADP, to form ATP and 3-phosphoglycerate
Step 6
Energy from the exergonic reaction is used to attach a phosphate group to the oxidized substrate.
G3P is oxidized by the transfers of electrons to NAD+, creating NADH
Energy Investment Phase
Step 5
Conversion between DHAP and G3P
Step 4
Aldolase cleaves the sugar molecule into 2 difference (3) carbon sugars
Step 3
Phosphofructokinase transfers a phosphate group from ATP to the opposite side of the sugar, investing a ATP.
Step 2
Glucose - 6 phosphate us converted to frutose 6 phosphate
Step 1
Hexokinase transfers a phosphate group from ATP to glucose
![True Jarrel [STUDENT] True Jarrel [STUDENT]](https://cdn1.mindomo.com/resources/img/about/mindomo-logo.png)