Oxidation & Reduction are opposite and reversible reactions of each other.
Condensation & Hydrolysis are opposite and reversible reactions of each other.
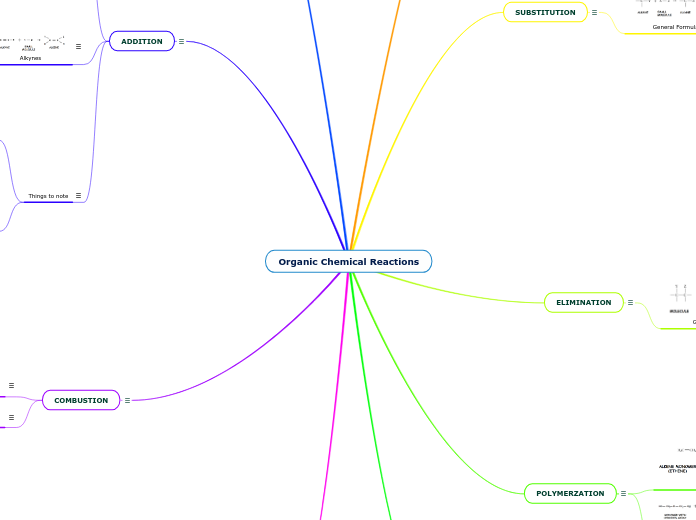
Organic Chemical Reactions
CONDENSATION
Condensation reactions are reactions in which two large molecules combine to form one larger molecule and one small molecule. The small molecule is usually water.
Condensation reaction of a large molecule and another large molecule producing a larger molecule and water.
One of the large molecules must contain a hydroxyl group to create water!
R can be any of the carbon derivatives.
OH+HO
Condensation reaction where two hydroxyl groups bond and produces a large molecule and water. The oxygen from one of the hydroxyl groups stays on the larger molecule.
Esterification (ester)
ESTERIFICATION:
Reaction of a carboxylic acid and alcohol to produce an ester and water.
The reaction is catalyzed by a strong acid.
Butanoic Acid + Butanol --> Butyl Butanoate + Water
Esterification (condensation) reaction of Butanoic Acid and Butanol produces Butyl Butanoate and water.
The reaction is catalyzed by a strong acid.
H+HO
Condensation reaction where a hydrogen bonds to a hydroxyl group and produces a large molecule and water
Amide
Reaction of a carboxylic acid and ammonia or amine to produce an amide and water.
Butanoic Acid + N-methylethanamine --> N-ethyl-N-methylbutanamide + water
Condensation reaction of Butanoic Acid and N-methylethanamine produces N-ethyl-N-methylbutanamide and water
COMBUSTION
Combustion reactions are reactions in which a compound reacts with oxygen to produce the oxides of the elements that produce that compound.
In the case for organic compounds it is generally hydrocarbons that react with oxygen.
Incomplete Combustion
In an incomplete combustion reaction, a Hydrocarbon reacts with oxygen Gas to produce Carbon Dioxide, Carbon Monoxide, Soot (unburned carbon), Water, and releases energy.
Pentane + Oxygen Gas --> Carbon + Water + energy
Incomplete combustion of Pentane and Oxygen Gas producing Carbon(soot), Water, and releases energy.
Complete Combustion
In an incomplete combustion reaction, a Hydrocarbon reacts with oxygen Gas to produce Carbon Dioxide, Water, and releases energy.
Pentane + Oxygen gas --> Carbon Dioxide + Water + energy
Complete combustion of Pentane and Oxygen Gas producing Carbon Dioxide, Water, and releases energy.
ADDITION
Addition reactions are reactions in which atoms are added to the double bonds of alkenes or the triple bonds of alkynes. If the carbon in the product is attached to more atoms then the reaction is an addition reaction.
Things to note
Some things to note are the Markovnikov rule and the isomers that may form.
When a small molecule with different atoms is added to an asymmetric alkene, isomers can form depending on which atom gets bonded to which carbon.
Pent-2-ene + Hydrochloric Acid -->
Reaction of Pent-2-ene and Hydrochloric Acid produces produces 2-chloropentane or 3-chloropentane
2-chloropentane
2-chloropentane is produced because the chlorine atom bonded to the second carbon
3-chloropentane
3-chloropentane is produced because the chlorine atom bonded to the third carbon
Markovnikov's Rule
Markovnikov's Rule states that when a small molecule is added to an asymmetric alkene, the hydrogen atom of the small molecule will attach to the double carbon bond that already has the most hydrogen bonds. This is also known as the 'rich gets richer'.
Propene + Hydrochloric Acid --> 2-chloropropane
Hydrohalogenation reaction of Propene and Hydrochloric Acid produces 2-chloropropane.
The Hydrogen from the Hydrochloric Acid will attach to the middle carbon as it only has 1 carbon-hydrogen bond
Alkynes
An addition reaction with an alkyne and a small molecule will produce an alkene with the small molecule attached. The small molecule is generally H2O (l), H2, HX, or X2 (where X = F, Br, Cl, or I).
MOST OF THE TIME THE ALKENE WILL IMMEDIATELY TURN INTO AN ALKANE UNLESS THERE IS A LIMITED AMOUNT OF THE SMALL MOLECULE.
The subtypes for Alkenes also apply similarly to Alkynes (Halogenation, Hydration, etc.)
Excess
When the small molecule is in excess the Alkyne will turn into an Alkene and then into a Alkane.
But-2-yne + Bromine Gas (excess)--> 2,3-dibromobutane
Halogenation reaction of But-2-yne and excess Bromine Gas produces
2,3-dibromobutane
Limited
When the small molecule is limited the Alkyne will turn into an Alkene.
But-2-yne + Bromine Gas (limited) --> 2,3-dibromobut-2-ene
Halogenation reaction of But-2-yne and limited Bromine Gas produces
2,3-dibromobut-2-ene
Alkenes
An addition reaction with an alkene and a small molecule will produce an alkane with the small molecule attached. The small molecule is generally H2O (l), H2, HX, or X2 (where X = F, Br, Cl, or I).
Halogenation (Dihaloalkane)
HALOGENATION:
Addition of an Alkene and Halogen Gas to produce a DiHaloAlkane. (where X = F, Br, Cl, or I).
But-2-ene + Bromine Gas --> 2,3-dibromobutane
Halogenation reaction of But-2-ene and Bromine Gas producing
2,3-dibromobutane
Hydrohalogenation (Haloalkane)
HYDROHALOGENATION:
Addition of an Alkene and Binary Acid or Hydrogen Halide to produce a HaloAlkane. (where X = F, Br, Cl, or I).
But-2-ene + Hydrochloric Acid --> 2-Chlorobutane
Hydrohalogenation reaction of But-2-ene and Hydrochloric Acid producing
2-Chlorobutane
Hydration (Alcohol)
HYDRATION:
Addition of an Alkene and Water to produce an Alcohol.
But-2-ene + Water --> Butan-2-ol
Hydration Reaction of But-2-ene and Water producing Butan-2-ol.
Hydrogenation (Alkane)
HYDROGENATION:
Addition of an Alkene and Hydrogen gas to produce an Alkane.
There is generally a catalyst in this reaction and the reactants are under heat and pressure.
General formula:
CnH(2n) + H2 --> CnH(2n+2)
But-2-ene + Hydrogen gas --> Butane
Hydrogenation reaction of But-2-ene and Hydrogen gas producing Butane.
C4H8 + H2 --> C4H10
REDUCTION
Reduction reactions are reactions in which a carbon atom forms less bonds with oxygen, or more bonds with hydrogen. They can also be classified as an addition reaction.
Reduction reaction with a molecule that has a double bond and a reducing agent produces a molecule with a single bond, with more total hydrogen bonds
all double/triple bonds
Reduction reactions also apply to triple bonded carbon.
Alkyne + Reducing Agent --> Alkane
Reduction reaction of an Alkyne and a Reducing Agent producing an alkane.
Can also be done with an alkene instead of alkyne
Ketone
Reduction reaction of a Ketone and a Reducing Agent producing a Secondary Alcohol.
Butan-2-one + Reducing Agent --> Butan-2-ol
Reduction reaction of Butan-2-one and a Reducing Agent producing Butan-2-ol
Reduction reaction of an Aldehyde and a Reducing Agent producing a Primary Alcohol.
Propanal + Reducing Agent --> Propanol
Reduction reaction of Propanal and a Reducing Agent producing Propanol
HYDROLOSIS
Hydrolysis reactions are reactions in which one larger molecule and one small molecule combine to form two large molecules The small molecule is usually water.
Hydrolysis reaction of a larger molecule and water producing a large molecule and another large molecule.
One of the large molecules must contain a hydroxyl group to create water!
R can be any of the carbon derivatives.
Hydrolysis of Ester
Hydrolysis of an ester:
Reaction of an ester and water to produce a carboxylic acid and alcohol.
The reaction is catalyzed by a strong acid.
Butyl Butanoate + Water --> Butanoic Acid + Butanol
Hydrolysis reaction of Butyl Butanoate and water produces Butanoic Acid and Butanol.
The reaction is catalyzed by a strong acid.
POLYMERZATION
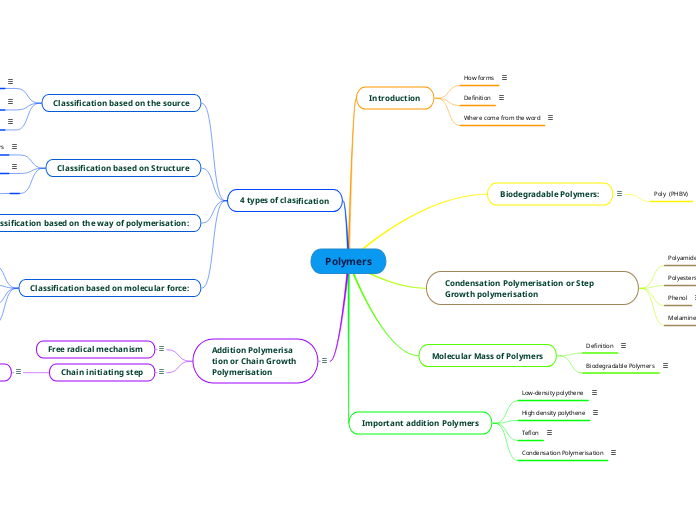
A Polymer is a long chain of repeating small molecules called monomers.
Condensation
Polymer reaction in which two Monomers (One must contain a hydroxyl group) combine to form Polymers and Water through a series of condensation reactions.
Terephthalic Acid + Ethane-1,2-diol --> Polyethylene Terephthalate + Water
An addition polymerization reaction of Terephthalic Acid and Ethane-1,2-diol producing Polyethylene Terephthalate and Water
Addition
Polymer reaction in which Alkene Monomers combine to form Alkene Polymers through a series of addition reactions.
Styrene + Styrene --> Polystyrene
An addition polymerization reaction of Styrene producing Polystyrene
ELIMINATION
Elimination reactions are reaction in which atoms are removed from a molecule to form a double bond
An elimination reaction of a molecule losing two atoms and producing a molecule that contains double bonds and another small molecule
Isomers
When an asymmetric alkane undergoes an elimination reaction, they may create multiple isomers.
2-Chlorobutane + Sodium Ethoxide -->
[But-2-ene AND But-1-ene] + Ethanol + Sodium Chloride
Dehalogenation elimination reaction of 2-Chlorobutane and Sodium Ethoxide producing [But-2-ene AND But-1-ene], Ethanol, and Sodium Chloride
Dehydration
DEHYDRATION:
Elimination reaction in which a Secondary Alcohol produces an Alkene and Water
This reaction contains a catalyst, H2SO4
Rectangle = -->
Butan-2-ol --> But-2-ene + Water
Dehydration elimination of Butan-2-ol producing But-2-ene and Water.
This reaction contains a catalyst, H2SO4
Dehalogenation
DEHALOGENATION:
Elimination reaction in which a Haloalkane and Sodium Ethoxide Produces an Alkene, Ethanol, and a Sodium-Halogen molecule.
2-Chlorobutane + Sodium Ethoxide --> But-2-ene + Ethanol + Sodium Chloride
Dehalogenation elimination reaction of 2-Chlorobutane and Sodium Ethoxide producing But-2-ene, Ethanol, and Sodium Chloride
SUBSTITUTION
Substitution reactions are reactions in which hydrogen atoms or a functional group is replaced by a different functional group.
General Formula
Substitution reaction of an alkane and a small molecule producing an alkane and another small molecule with the atoms swapped.
Multiple Substitutions
A substitution reaction can repeat many times until there are no available hydrogens to be swapped
Alkane example
Substitution reaction of an Alkane and Halogen producing an Alkane with the Halogen attached and a Binary Acid
After repeating again, the product in the first reaction reacts with the Halogen and creates a new alkane, with two of the halogen atoms attached
Benzene & Cycloalkanes
Benzenes and Cycloalkenes behave similarly to alkanes and will substitute their hydrogen atoms for other halogens.
Cycloalkane
Substitution reaction of a cycloalkane and halogen gas producing a cycloalkane with the halogen attached and a binary acid.
Cyclohexane + Chlorine Gas --> Chlorocyclohexane + Hydrochloric Acid
Substitution reaction of Cyclohexane and Chlorine Gas producing Chlorocyclohexane and Hydrochloric Acid
Benzene
Substitution reaction of Benzene and a Halogen Gas Producing a Benzene with the halogen attached and a Binary Acid.
THE HALOGEN MUST EITHER BE CHLORINE OR BROMINE AND NEEDS A STRONG CATALYST, FeCl3 AND FeBr3 RESPECTIVLY.
Benzene + Chlorine Gas --> Chlorobenzene + Hydrochloric Acid
Substitution reaction of Benzene and Chlorine Gas, producing Chlorobenzene and Hydrochloric Acid.
This reaction needs a strong catalyst, FeCl3
Alkane
Hydroxyl Group for Halogen
Substitution reaction of an alcohol and a binary acid producing an alkane with the halogen and water.
Butanol + Hydrochloric Acid --> Chlorobutane + Water
Substitution reaction of Butanol and Hydrochloric Acid producing Chlorobutane and water.
Halogen for Hydroxyl Group
Substitution reaction of an alkane and a hydroxide ion producing an alcohol and a halogen ion.
Chlorobutane + Hydroxide Ion --> Butanol + Hydrochloric Acid
Substitution reaction of Chlorobutane and a Hydroxide ion producing Butanol and Hydrochloric Acid.
Hydrogen for Halogen
Substitution reaction of an alkane and a halogen producing an alkane with the halogen and a binary acid.
Butane + Chlorine Gas + Chlorobutane + Hydrochloric Acid
Substitution reaction of Butane and Chlorine gas producing Chlorobutane and Hydrochloric Acid.
OXIDATION
Oxidation reactions are reactions in which a carbon atom forms more bonds with oxygen, or less bonds with hydrogen. They can also be classified as an elimination reaction.
Aldehyde
Oxidation reaction of an Aldehyde and an Oxidizing Agent producing a Carboxylic Acid
Propanal + Oxidizing Agent --> Propanoate
Oxidation reaction of a Propanal and an Oxidizing Agent producing Propanoate
Secondary Alcohol
Oxidation reaction of a Secondary Alcohol and an Oxidizing Agent producing a Ketone AND WATER
Butan-2-ol + Oxidizing Agent --> Butan-2-one
Oxidation reaction of a Butan-2-ol and an Oxidizing Agent producing Butan-2-one AND WATER
Primary Alcohol
Oxidation reaction of a Primary Alcohol and an Oxidizing Agent producing an Aldehyde AND WATER
Propanol + Oxidizing Agent --> Propanal
Oxidation reaction of a Propanol and an Oxidizing Agent producing Propanal AND WATER