af adja sylla 3 år siden
166
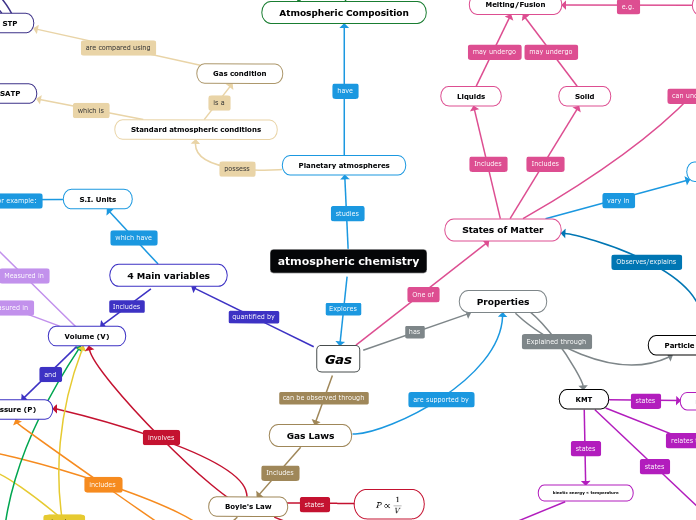
atmospheric chemistry
In the study of gases, several key principles and variables are crucial for understanding their behavior and properties. Gases are highly compressible and do not have a fixed shape, adapting to the volume of their containers.