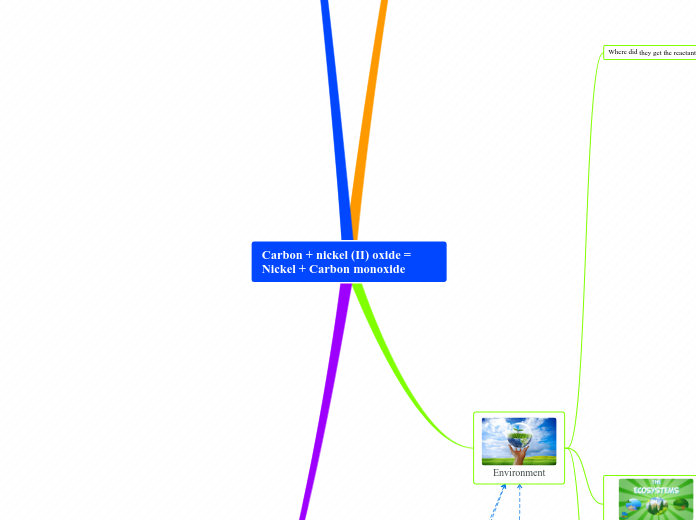
Carbon + nickel (II) oxide = Nickel + Carbon monoxide
Society
Product in this reaction
Nickel + Carbon monoxide
Annual production of this reaction
The annual production of this reaction is an estimated 2.2 million tones in the current year
Cost associated with this reaction
Carbon monoxide
A lab bottle is the smallest size you can buy, Cost should be under $150-$250 if you don't need medical-grade purity, plus ground shipping.
The more you buy, the cheaper it is.
the cost of Nickle associated to this reaction Nickel 11,457.00 USD per Ton
Government policies
The government related policy are
National Environmental Policy Act (
Clean Water Act
Toxic Substances Control Act
Comprehensive Environmental Response, Compensation, and Liability Act
Health concerns
Higher exposures can cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency, with severe shortness of breath.
May cause cancer
Breathing Nickel Carbonyl can irritate the lungs causing coughing and/or shortness of breath.
Causes damage to organs through prolonged or repeated exposure
May damage fertility or the unborn child Danger Reproductive toxicity
Exposure can cause headaches, dizziness, nausea and confusion.
Harmful if swallowed
May cause allergy or asthma symptoms or breathing difficulties if inhaled
How is it useful?
used to get a pure nickel, which can be used in a variety of applications. For rechargeable batteries, it is used in a powder form and in the battery it combines with other metals to form a reaction.
Carbon monoxide is a very important industrial compound. In the form of producer gas or water gas, it is widely used as a fuel in industrial operations.
This reaction is used to get pure nickel, which can be used in a variety of applications.
Example
Cutlery
Jet engines
Mobile phones
Cars
Coins
Batteries
Bathroom taps and shower heads
Sciences
Is the reaction part of a larger series
yes the reaction is part of a larger series than An element that is higher in the series will displace an element that is below it.
Energy Requirements
An exothermic reaction is a chemical reaction that releases energy through light or heat. It is the opposite of an endothermic reaction
Product
Carbon Monoxide s a highly poisonous, odorless, colorless, and tasteless gas. It is very flammable in air over a wide range of concentrations
Solid, Slivery-white metal, good conductor, Ductility, Malleability,Luster,Hardness
Reactants
Nickle Monoxide
Properties
Nickel oxide appears as odorless green-black cubic crystals (yellow when hot) or green powder
carbon
properties
Black, non-metal, conductor
Density- 2.2 g.cm-3 at 20°C
Melting Point- 3652 °C
Boiling point- 4827 °C
Purpose Of Reaction
Nickel reacts with the carbon monoxide, forming nickel carbonyl, a gas
This reaction converts nickel oxides into nickel metal with very high purity being attainable in just a single process.
To extract and purify nickel.
types of reaction
Single Displacement
Balanced Equation
C+Ni2 O = 2Ni + CO
Word Equation
Environment
Waste disposed
Recycling Carbon Monoxide can be put to good use as it could be used in the future as commercial purposes as in metals, medicines, and food while others can be converted into energy.
Nickel
Recycling is an important factor in nickel's life cycle and an important contributor to global sustainability.
How does it impact the ecosystem
Carbon Monoxide
Increasing storm activity and causing other extreme weather events
Related to the sea temperature increases changing to ecosystems
Linked with climate change and global warming
Affects the number of green house gasses
Environmental impact
Nickle
Habitat destruction
Greenhouse gas emissions
Contamination of the air, water and soil
Where did they get the reactants from
Carbon
Rocks
Soil
Atmosphere
Nickle monoxide
Nickle is mined form industrial
Technology
How is the technology efficient
Technology used in this reaction
Nickel MOCVD Process
The process is recognized as the most environmentally friendly metal refining process by the governments of Canada and the United States
Nickel carbonyl refining process is approved by the environmental monitoring branches of the governments of Ontario, Canada
Very good uniformity of deposition.
Low residual stress
nickel alloys can be controlled during the deposition
High deposition rate
Ultraline Cylinder Package
the Ultraline cylinder package is designed to minimize metal carbonyl impurities in CO by using an iron and nickel free container.
Benefit
Reduced water-contamination risk
Quicker to install
Balanced pressure at outlets