Wash your hands before leaving
Dilute acid or base spills
No eating
Report any faulty equipment to the teacher
Rinse if a chemical comes in contact with your eyes or skin
Aim test tube mouths away from people
Safety goggles must be worn at all times during experiments
Begin experiment only when you are instructed to
Use a fume hood when instructed to do so
Keep work area clean
Know where all safety equipment is and how to use it
Do not mix concentrated acids or bases
Clean up all chemicals after activity
Do not return unused chemicals to bottles
Report any accident to the teacher
Be careful when handling hot objects
Follow teacher's instruction thoroughly
Do not taste any substance
No loose hair or clothing
Contact lenses should not be worn
Read each activity thoroughly before completing, if you do not know how to do something consult with teacher
No unauthorized experiments
Scientific Method
Question
Ask a question about something that interests you
It must be simple, specific, and do-able, -comparing 2 things
Example: Does the amount of exercise affect the heart rate?
Does eating while driving affect your driving skills?
Hypothesis
Format If_____ Then_____
An educated guess about what you think will happen
Does not need to be correct
Example: If you exercise then your heart rate will increase
If I eat while I drive then my driving skills will become better
Variables
Example: Does the amount of caffeine ingested
affect a person's heart rate?
Hypothesis: If a person ingests more than
5MG of caffeine, then the heart rate will increase
Independent Variable: The amount of caffeine
Dependent Variable: The heart rate
Controlled variable: Time of day, watch, person measuring, age
Independent: What I change
Dependent: What I observe
Controlled: What I keep the same
Variables
Types of Variables
Controlled Variable (CV)
All the other things are kept the same
Dependent Variable (DV)
The variable that you measure-The effect
Independent Variable (IV)
The Variable that you change-The cause
Procedure
Step by step description of how the experiment is conducted
Avoid the use of "he" or "she" or "I"
Number the steps 1 2 3
Can also use diagram of set-up
Observations
Make careful notes of everything you observe during the experiment
Record data in graph, table or chart form
Steps of Inquiry
Question
Hypothesis
Variables
Procedure
Observations
Discussion/Analysis
Conclusion
Discussion/Analysis
Identify patterns or trends in your data
Develop a possible explanation
Answer any questions with the lab
Conclusion
State whether your results are supported, partially supported, or rejected your hypothesis
Suggest possible sources of error
Suggest possible improvements
Safety
Two types of safety symbols
WHMIS
There are 8 WHMIS symbols
Each WHMIS symbol has a circle for its border
Workplace Hazardous Materials Information System
Found on workplace products-mostly clearers
HHPS
Hazardous Household Product Symbol
Found on household products-mostly cleaners
Hazardous Safety Symbols
Safety Symbols: Have been developed to warn users of the hazards associated with different products.
Hazardous Houshold Product Symbols
Caution: Yellow triangle Warning: Orange diamond Danger: Red Hexagon
Combining the type and level of hazards creates HHPS
Three levels: There are 3 levels of hazards, each more harmful than the previous
Four types: There are 4 different types of hazards
Poisonous, Explosive, Corrosive, Flammable
There are 12 HHPS representing different types and levels of hazard
Acids and Bases
The pH scale measures how acidic or basic a solution is
The strength of an acid or base may be measured on the pH scale. The scale runs from 0-14
Example: an acid that is a pH 5 is 10 times stronger than an acid of pH 6 etc.
Strong and Weak
Some acids and bases are much stronger (Have a high concentration of ions in solution than others)
Eg: Nitric acid is a strong acid; ammonia is a weak base
Examples of Bases
Ammonia
NH3
Sodium Bicarbonate
NaHCO3
Aluminum Hydroxide Al(OH)3
Calcium Hydroxide
Ca(OH)2
Sodium hydroxide
NaOH
Examples of Acids
Hydrochloric Acid (In your digestive system)
HCL
Carbonic Acid (In soft drinks)
H2CO3
Citric Acid (In citrus fruit)
H3C6H5O7
Acetic Acid (in Vinegar)
HC2H3O2
Water is neutral (7)
Anything above 7 is a base
The closer the pH is to 7 the weaker the base
Anything below 7 is an acid
The closer the pH is to 7, the weaker the acid
Indicators
It is possible to test the pH of a solution using indicators, eg: litmus paper
Red litmus paper turns blue in a base and remains red in neutral or acidic solutions
Blue litmus paper turns red in an acid and remains blue in neutral or alkaline solutions
Properties of Acids
Tastes sour
Are corrosive (reacts with metals to produce hydrogen gas)
Release hydrogen ions (H+) in solution
Naming Acids
(hydrogen + a single element) are called hydro__ic acids
Eg: H2S is Hydrosulphuric acid
Properties of Bases
Taste bitter
Feels slippery
Are corrosive (break down proteins)
Release hydroxide ions (OH-) in solution
Rules for writing ionic formulas
Write symbols of two elements
Write valence of each as superscripts
Drop positive and negative signs
Criss-cross the superscripts so they become subscripts
Reduce when possible
Bonding
Covalent
Electrons are shared between non-metals
Most transition metals have multiple valences
Roman numerals are used in the name of transition metal in the compound to show the valence on the cation (metal)
Neither takes a charge
Do not criss-cross
USE prefixes in name
Name tells formula
Cannot reduce
Binary Ionic
Ion= when an element has more protons than electrons or vice versa
Non-metal becomes a negative ion (anion) gains electrons from metal
Chlorine gains electron to fill valence shell [Cl]-
Metal in bond becomes a positive ion (cations) loses electrons to non-meta
Lithium loses valence electron and becomes [Li]+
Magnesium loses two valence and becomes [Mg]2+
Occurs between metal and non-metal
Coefficient
shows how may molecules there are of a particular chemical
EX: 3 H2O
Means there are 3 water molecules
Subscript
shows how many atoms of an element are in a molecule
Ex: H2O
2 atoms of hydrogen (H)
1 atom of oxygen (O
Law Of Conservation of Mass
If an equation obeys the Law of Conservation it is balanced
In a chemical reaction, matter is neither created nor destroyed
In other words, the number and type of atoms going INTO a reaction must be the same as the number and type of atoms coming out.
Rules
Subscripts cannot be added, removed, or changed
Coefficients can only go in front of chemical formulas...NEVER in the middle of a formula
You can only change coefficients
Matter cannot be created or destroyed
Tips
Try balancing big things first, save free elements for last
There is no one way to do it properly.
If the same polyatomic ion appears on both side of the equation, it's usually okay to treat it as one unit
Chemical Equation
Parts of an equation
Reactant ---> product
Reactants react to produce the product
Describes a chemical change.
Reactants and Products
Reactant
Written on the left side of equation
The chemical (s) you start with before the reactions.
Product
Right side of the equation
The new chemical (s) formed by the reaction
Diatomic Elements
Always found in pairs when alone as an element
H O F Br I N Cl
Balancing Chemical Equations
Combustion
Usually a mix of Oxygen, Carbon, Hydrogen, and energy (heat or light)
Example: CH4 + O2 ----> CO2 + H2O
Single Displacement
A + BC -----> B + AC
(Elements switch partners to form new compounds {zinc bumps hydrogen out to form zinc chloride})
Example: 2HCl + Zn ---> ZnCl2 + H2
One element knocks another element out of a compound
Double Displacement
AD + BC ---> BD + AC
(Compound swap partners)
Example: BaCl2 + Na2SO4 ---> BaSO4 + 2NaCl
Two compounds switch with each other
Decomposition
AB -----> A + B
(Compound breaks to = two elements)
Example: 2H2O2 ----> 2H2O + O2
One substance breaks down into two or more substances
Synthesis
A + B ---> AB
(element + element = compound)
Example: 2Na + Cl2 --> 2NaCl
Two or more chemicals bond together forming one new substance
Chemical Reactions
Neutralization
Addition of an acid or alkali (base) to a liquid to cause the pH of the liquid to move towards a neutral pH of 7
Base
Adds Hydroxyl ions
OH-
Sodium Hydroxide
NaOH ---> Na+ + OH-
Acid + Base =
H+ + OH- ---> H2O
HCl+ NaOH ---> H2O + NaCl + energy
Acid + base ---> water + salt + heat
NEUTRAL
Acid
Adds Hydrogen ions
H+
Hydrochloric acid
HCl ---> H+ + Cl-
Nomenclature
Naming/Prefixes
Mono
Di
Tri
Tetra
Penta
Hexa
Repta
Octa
Nona
Deca
Valence Electrons
Electrons in the outermost shell
Group 1 has 1 valence E and group 2 has 2 valence E and so on
Valence electrons are determined by element group
Groups are the vertical columns on the table
Florine has 7 valence electrons
Lewis Dot
Draw chemical symbol
Determine valence electrons
Skip middle (transition metals) when counting groups
Draw valence electrons around symbol
Skip transition metals they have weird rules
Draw symbol and then the valence electron(s)
Bohr Diagram
Neutrons= atomic mass – # of Protons
Orbit 1- 2 Electrons
Orbit 2- 8 Electrons
Orbit 3- 8 Electrons
Protons and Neutrons go in the nucleus
Draw Nucleus
Draw total number of electrons in correct orbit arrangement
A model of an atom with the nucleus at the center, and the electrons drawn around it on different energy levels or electron orbits
Renewable Energy
Geothermal
Biomass
Wind
Hydro
Tidal
Solar
How can we reduce climate change
Ban burning of carbon
Recycle, Reuse, Reduce
Not over farm
Ride a bike/walk instead of drive
Build up instead of out
Consequences of climate change
Rising oceans
Melting ice caps
Heat
Extreme cold
Climate refugees
Dying Crops
Extreme storms
Activities that contribute to climate change
Air pollution
radioactive waste
Plastic
Deforestation
Burning of fossil fuels
Climate change is the change of the Earth's or a regions weather patterns. (Extreme weather)
Cardiac
Makes up heart which pumps blood
Does not tire
Branching
Found only in heart
attaches to other cardiac muscle cells
Skeletal
quick to respond, quick to tire
Moment is main function
Non-branching
Long muscle fibres
attached to bones by tendons
Striped
Triceps, Biceps, Quadriceps
Smooth
Contracts hollow organs
Controlled by central nervous system and hormones
Lining of digestive, urianary, and reproductive systems
Slower to respond slower to tire
Not striped
Why We Breathe
Get oxygen to cells
Remove CO2 from cells.
Air Pathway
Digestive
Rectum
Muscular tube
Holding area
Small Intestine
Pancreatic fluids
Amylase
Lipase (breaks down fat)
Pepsin
Sodium bicarbonate
Other enzymes
Bile added
Nutrient absorption
Chemical digestion
Large Intestine
Production of vitamin K and biotin by bacteria
Reabsorption of water into the body
What are nutrients?
Glucose, starch
Fatty acids + glycerol, fats
Amino acids, proteins
Stomach
Now called chyme
Food stays for 4-5 hours
Lined with mucus
Gastric Fluids
Water
Mucin
Pepsin (breaks down protein)
HCL
Physical and Chemical digestion
Epiglottis
Covers passage to trachea
Esophagus
Moves food to stomach by peristalsis
No digestive occurs
Bolus
Flexible, muscular tube
Mouth
Saliva –amylase (breaks down starch)
Teeth and tongue
Physical and chemical breakdown
Stages of Digestion
Elimination- Feces held in rectum until ready
Absorption- Small intestine for nutrients, large intestine for water
Digestion-
Mechanical (mouth and stomach)
Chemical (mouth stomach small intestine)
Ingestion- Mouth
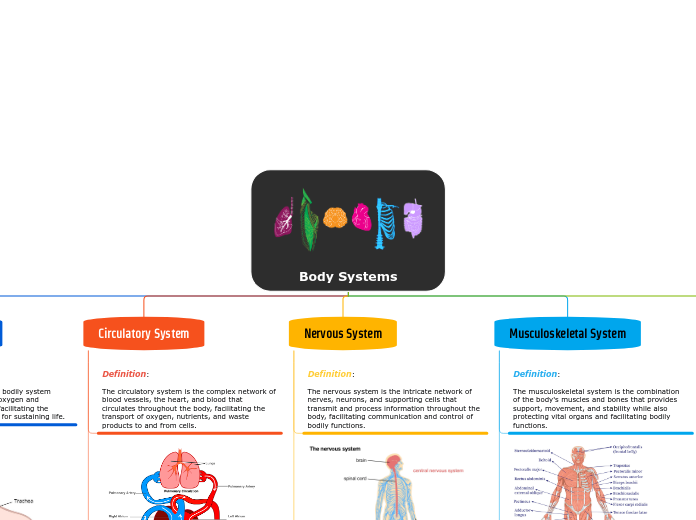
Respiratory
Respiratory Epithelial Cells
Helps move mucus and filter foreign particles
Produce Mucus
Have Cilia
Mouth/Nose
Larynx
Trachea
Left/right Bronchus
Bronchioles
Alveoli
Breathing
Exhale
Ribs contract
Diaphragm dome-snapped (pushes up) muscles relax
Inhale
Ribs expand
Diaphragm flattens muscles contract
Gas Exchange
Occurs at the alveoli
Carbon Dioxide leaves the blood by DIFFUSION
Oxygen enters the bloodstream in the lungs by DIFFUSION
Epiglottis covers esophagus when you swallow to prevent food from going down throat
Muscular
Circulatory
Heart
Has valves and veins
Has 4 chambers
Three Main Features
Vessels- where blood flows through
Pump- pumps the fluid through vessels
Fluids- transports materials (blood)
Blood Vessels
Capillaries: Allow exchange of nutrients, wastes, and gasses.
Veins: Carry blood to heart
Arteries: Carry blood away from heart
Mitosis
Prophase
Spindle fibers being to appear
Nuclear membrane disappears
Chromatin condenses into chromosomes
Metaphase
Spindle fibers attach to the centromere
Chromosomes migrate to the middle of the cell (equator)
Anaphase
Cleavage furrow starts to form in animal cell and a cell plates forms in plant cells
Chromosomes are pulled apart "away" from sister chromatid
Interphase
Consists of 3 part
Resting and normal cellular function
Cells spend most of its life in this phase
Telophase
Cleavage furrow/cell plate closes off to create two identical sister cells
Cytoplasm and organelles duplicate in a process called cytokinesis
Cells
Plant Cells
Square shaped
Cell Membrane
Regulates what enters and leaves the cell
Nucleolus
Makes RNA and Ribosomes
Cell Wall
Found in plant cells
Provides protection to cell
Smooth Endoplasmic Reticulum
Helps move lipids and steroids
Rough Endoplasmic Reticulum
Folds proteins to correct shapes
Nuclear Membrane
Separates nucleus contents from rest of cell
Nucleus
Stores cell DNA and controls cell
Animal Cells
Round
Lysosomes
contains digestive enzymes
Golgi Apparatus
processes proteins
Ribosomes
Make proteins, crucial to cell devision
Cytoplasm
Gives cell its shape. Keeps organelles in place
Chloroplast
Found in plants
Coverts light energy into sugars
Mitochondria
Cellular Resperation
Breaks down nutrients and turns it into energy
Vacuole
Largest organelle in plant cells
Holds Materials and waste.
Lenses
Concave
Often called a diverging lens because when surrounded by material with a lower index of refraction, rays passing through it spread out
Thinner in the middle than at the edges
A lens is a piece of transparent material, such as glass or plastic, that is used to focus light and form an image
Convex
Often called a converging lens because when surrounded by material with a lower index of refraction, it refracts parallel light rays so that the rays meet at a point
Is thicker at the center than at the edges
Index of Refraction
Index of refraction = Speed of light in space
Speed of light in media
Cannot be less than 1.00
Air=1.00 Glass=1.5 Water= 1.33
As light travels from one medium to another, the speed of light changes and the light bends accordingly.
You will have no trouble remembering this if you think about it in the right way: a real image has to be where the light is, which means in front of a mirror, or behind a lens.) Virtual images are formed by diverging lenses or by placing an object inside the focal length of a converging lens.
Optical Images
Type: Real or Virtual
Size: Bigger smaller, or same size as object
Orientation: Vertical (Upright or inverted) Lateral (Left to right)
Location: Behind or in front of the mirror
An image is the representation of an object formed by the interaction of light rays.
Diagrams
Referred to as Ray Diagrams
A beam is a 'bundle' of light rays
A ray is a single path followed by light
Laws
The incident ray, reflected ray, and normal all lie on the same plane
The angle of incidence is equal to the angle of reflection
Plane Mirrors
Characteristics
Type: Virtual
Size: Same size object
Orientation: Image is upright, and laterally inverted
Location: Behind the mirror the same distance from the mirror as the object
Light Travels
In a straight line
This is known as Law of Rectilinear Propagatio
Reflections
Diffuse Reflections
Walls floors ceilings scatter light in all directions making them visible
Most objects are made of rough surfaces (and therefore scatter light); if this did not happen, indoor lighting would not be effective
They still obey the laws of reflection (Angle of incidence= angle of reflection)
Something that is flat will reflect nicely, something that is not flat will reflected not so nicely. (random like a broken mirror)
Because of the uneven surface, the light rays are scattered in many directions (diffused).
Many surfaces appear smooth, but when viewed under a microscope they are not.
Reflection of Light
Regular Reflection: of light from smooth shiny surfaces
Speed of Light
Types of Curved Mirrors
Convex Mirror
Behind Mirror
Upright
Smaller
Virtual
All images will be formed at the same place
Converging mirrors
A mirror whose reflecting surface curves inward
Concave Mirror
Diverging mirror
A mirror whose reflecting surface curves outward
Refraction
You can only have total internal reflection going from a high index of refraction to a low index of refraction
Snell's Law: The Law of Refraction
Index of refraction of the media in which the angle of refraction is
Index of refraction of the media in which the angle of incidence is
n1 sin θI = n2 sinθR
Curved Mirrors
Converge
To meet at a common point
Vertex
The point where the P.A. meets the mirror
Rules for the construction of ray diagrams
Hint: If the reflected rays of diverge (reflect AWAY from each other)
Trace the back behind the mirror to find a virtual image.
Rays striking V follow the laws of reflection (angle of incidence = angle of reflection)
Rays passing through C are reflected back along the same path
Rays passing through F are reflected parallel to the P.A.
Parallel rays are reflected through F
Focal Length (f)
The distance from the focal point to vertex
Focal Point (F)
Is halfway between the vertex and the centre of curvature. When parallel light rays are shone along the P.A., the reflected rays converge and cross at the focal point.
Principle Axis (P.A.)
The line through C that passes through the midpoint of the mirror (normal to the centre of the mirror
Centre of Curvature
Centre of a sphere whose surface has been used as a mirror
C=2f
Eye Anatomy
Subtopic
Cones
Active in high light levels. Able to see colour
Rods
Responsible for the vision at low light
Pupil
The hole in the middle of the iris where light enters the eye
Cornea
Clear area of sclera that helps bend light into the retina
Iris
Coloured sheet of muscle which controls the amount of light that enters the eye
Hyperopia
Lens type to fix: Converging
Far-sightedness
Myopia
Lens type to fix: Diverging
Near-sightedness
Vitreous Humour
Jelly-like fluid which helps keep the shape of the eyeball.
Optic Nerve
Responsible for sending information to the brain to be processed
Fovea
Small area of the retina which contains only cones
Retina
Layer of light sensitive cells which send impulses to the optic nerve
Sclera
White, tough outer part of the eye that provides protection
Lens
Clear disc which focuses the light onto the retina
Tribolumiscence
Produced by scratching or rubbing certain crystals
Fluorescence
An object absorbs UV light & immediately releases visible light
Production of visible light
UV hits fluorescent coating
Electric current causes Hg to emit UV
Fluorescent dyes in detergent, highlighter
Electrical Discharge
Production of light when electricity passes through a gas
Lightning, Neon signs
Gas atoms get excited and give off light
Incandescence
Production of light by heating to high temperatures
Gas Stove, Torch
Phosphorescence
Substances that contain "phosphors" that absorb light
Glow in the dark stuff
Light is emitted slowly
Bioluminescence
Light from living organisms
Fireflies
Chemiluminescence
Light from chemical reactions
Glow Sticks
Types of Light
Light
White Light
White light hits a prism at an angle the light disperses into its different wave lengths
Made up of a continuous sequence of colours
Light is a type of radiation, or electromagnetic energy that travels in waves
Sources of Light
Non-luminous objects: Reflect light
Luminous sources: Produce light
Science
Scientific Inquiry and Investigation
Optics
Chemistry
Climate Change
Biology