Three collagen polypeptides
Triple helix quaternary structure
Hydrogen bonds
Stable, strong, fibrous structure ideal for connective tissue
5-carbon sugar with aldehyde group on c-5
and hydroxyl groups on C-2 and C-3.
Two hydroxyl groups make it easier for ribose to participate in hydrolysis reactions
Nucleic Acids
Ribonucleic Acid
Deoxyribonucleic Acid (DNA)
Negatively charged phosphate group
Ribose sugar
Deoxyribose sugar
Phosphate group. C-5 on the sugar binds to oxygen on Phosphate.
Phosphodiester linkage
Nucleic acid backbone, comprised of phosphate and sugar
Nitrogenous bases, attached to C-1 on sugar molecule
Guanine, cytosine, adenine
Fourth base is thymine, specific to DNA
Hydrogen bonds are formed between complementary bases
Guanine binds with cytosine, adenine binds with uracil
One polynucleotide chain folds inwards on itself, bringing complementary bases on the same RNA strand close for hydrogen bonding.
Guanine binds with cytosine, adenine binds with thymine
Two separate DNA polynucleotide chains connect in antiparallel. Complementary bases on the two strands form hydrogen bonds.
Sequence of nitrogen bases code for the production of specific amino acids in the ribosomes.
Responsible for transcription of genetic information found in DNA and decoding of this information in the ribosomes.
Some viruses also use RNA as their primary unit for storing genetic information instead of DNA
Used for storing genetic information in most organisms
Found in the nucleus of cells in the form of chromosomes.
Fourth base is uracil, specific to RNA
Identical to ribose, with the oxygen atom from the hydroxyl group on C-2 absent.
Absence of a second -OH group makes DNA more stable, ideal for long-term preservation of genetic material
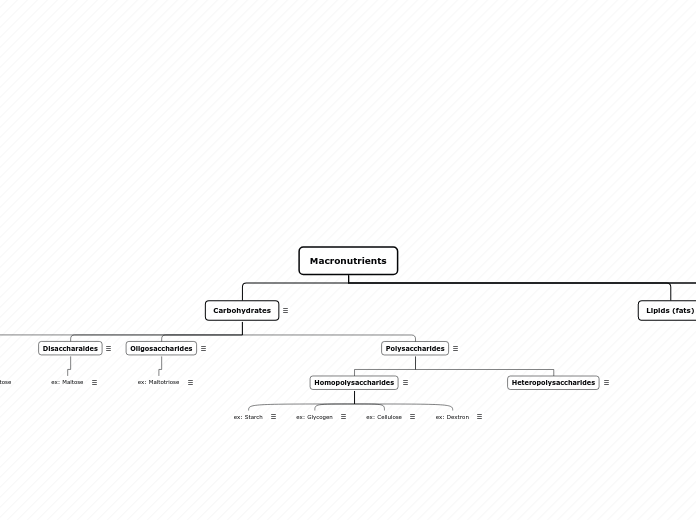
Lipids
Phospholipids
Cell membrane
Defines the structure of cells
Phospholipid bilayer structure
Amphipathic properties
of phospholipids
Triglycerides
Glycerol backbone
Three hydroxyl groups
Fatty acid carboxyl groups
Dehydration synthesis
Ester linkage
Two non-polar fatty acid chains
Polar phosphate group
Three fatty acid chains
Fats and oils
Take longer for the body to metabolize
than carbohydrates
Triglycerides are used for long-term
energy storage in plants and animals
Macronutrients
Proteins
Structural
Collagen
1/3 glycine amino acid
Very compact, dense animo acid
Closely packed, allowing for hydrogen bonding between collagen peptide chains
Connective tissue
Sensory
Rhodopsin protein
348 amino acids
Lysine amino acid residue
Receptor protein
Covalently bound
Light-sensitive molecule
Changes shape in response to light
Activation of other proteins in retinal rod cell
Informs the brain of the presence of light
Retina
Detect light
Made by animals for energy storage
High quantities of glucose in a compressed format makes for effective storage of glucose for later use in energy production.
Strong intermolecular
forces of attraction.
Glucose and water are both polar
Glucose is water soluble
Glucose can be dissolved and transported
within the body using water.
Simplest and smallest form of carbohydrate
Easy to break down for ATP production
Preferred source of energy for the body
Two polar substances in contact
Broken down into glucose and fructose by enzymes in the small intestine,
These monosaccharides can then be used in energy production.
Carbohydrates
Polysaccharides
Glycogen
Thousands of glucose monomers
Linked by 𝛂(1→4) glycosidic bonds.
Branches every 8-12 glucose units through an 𝛂(1→6) glycosidic bond.
Very large branched structures
Disaccharides
Sucrose
Polar and highly water soluble
Also contains carbonyl and hydroxyl
functional groups, enabling the creation
of hydrogen bonds
Plants make it as a form of
energy storage.
One glucose monomer and
one fructose (isomer of glucose)
monomer
Glycosidic bond between C1 on an 𝛂-glucose molecule and C4 on a 𝜷-fructose molecule.
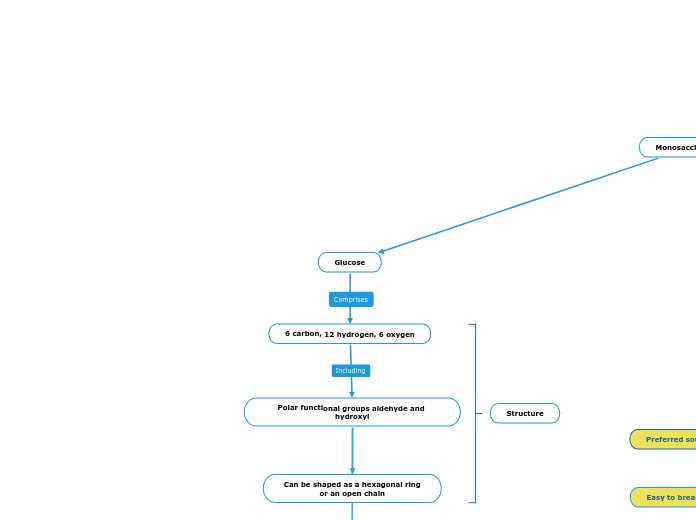
Monosaccharides
6 carbon, 12 hydrogen, 6 oxygen
Polar functional groups aldehyde and hydroxyl
Can be shaped as a hexagonal ring
or an open chain
Function
Structure
Additional info.
Glucose