von Webster Isha-Lee Vor 4 Jahren
351
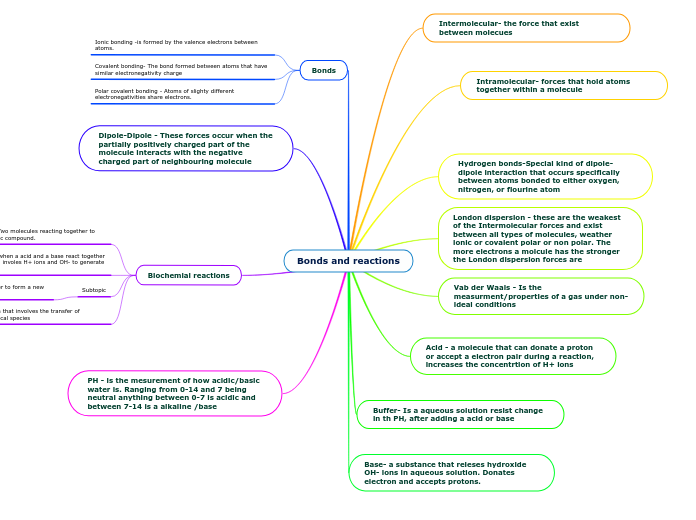
Bonds and reactions
Chemical bonding and reactions are fundamental concepts in chemistry. Different types of bonding include polar covalent, ionic, and covalent, each defined by how atoms share or transfer electrons based on their electronegativity.