door Webster Isha-Lee 4 jaren geleden
339
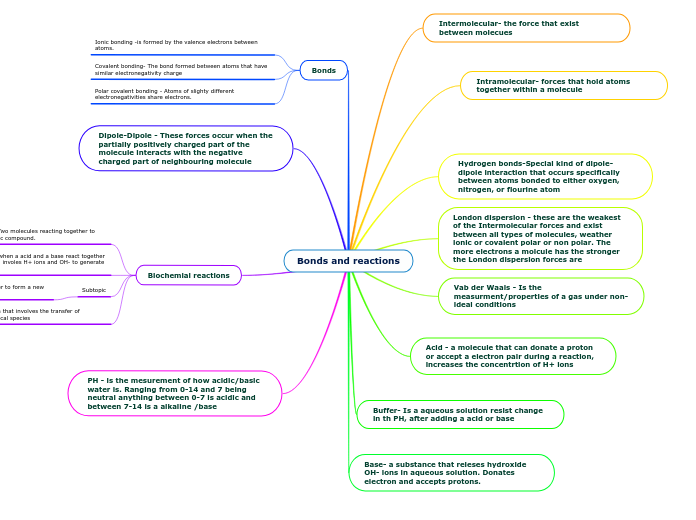
Bonds and reactions
Chemical bonding and reactions are fundamental concepts in chemistry. Different types of bonding include polar covalent, ionic, and covalent, each defined by how atoms share or transfer electrons based on their electronegativity.