FAVOURITE MEMORY FROM SCH4U
When you did experiments for us to watch,
also the balloon things to make us understand the structures. :)
Galvanic Cell Experiment
Elimination reaction
Loss of smaller molecule
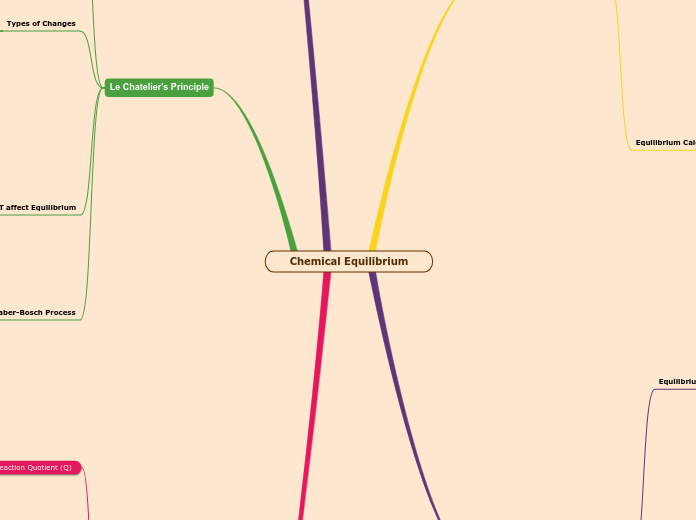
Equilibrium
Equilibrium Law
Rule of 100
Perfect Squares
Quadratics
Phase Equilibrium
Dynamic Equilibrium between
the physical states of
substances
Ie. Same molecule but change in state
Collisions can produce products
or reactants, the amount is equal
Chemical Reaction
Equilibrium
Dynamic Equilibrium
between the products
and reactants of a chemical
reaction
Le Chatelier's Principle
Any disturbance will be adjusted
by the system, occurs at equilibrum
Equilibrium Constant
Concentration at the
given equilibrium
Solubility Equilibrium
Dynamic equilibrium
between the solute and
solvent
Closed Systems
No change in matter,
energy can be exchanged
Reverse reaction
When the reaction goes
backwards (right to left)
Electrochemistry
Catalysts
Speed up a reaction
Oxidation States
Equals the charge of the atom
within the molecule
Corrosion
Ion is oxidized
Chemical Weathering
Cell Notation
Anode | Anode(aq) || Cathode(aq) | Cathode
Anode | Anode(aq) | Cathode
Standard Reduction Reaction
Tendency of something
to be reduced
Galvanic Cells
Spontaneous
reactions
Electrolysis
Redox Reactions
Half Reactions
Oxidizing Agent
Atom that gains
electrons
Reducing Agent
Atom that loses
electrons
Positive E, spontaneous
Negative E, non-spontaneous
Spectator Ions
Thermodynamics
Activation Energy
Energy needed for
a reaction to occur
Exothermic Reaction
A reaction that releases
heat
Endothermic Reaction
A reaction that absorbs heat
Gibbs Free Energy
∆G, The available
energy has to do work
Arrhenius Equation
The relationship between
the rate of reaction and
temperature for chemical reactions.
Collision Theory
a reaction consists of
particles that are always
randomly moving.
These collisions with other
particles/wall of container can cause
the breaking/forming of bonds
Brow nian Motion
Chemical Kinetics
Rate of Reactions
Rate Law
r = k[A]^m[B]^n
The RoR is related to the
[A] and [B] but not directly
proportional in all ways.
Average Rate
of Change (AROC)
Change over an
interval (2 points)
Instantaneous Rate
of Change (IROC)
Change at a
certain point
Catalysts!
Hess's Law
The total enthalpy
change is the sum of
all changes, regardless
of the # of steps/stages
of a reaction.
Enthalpy
∆H⌄x, The sum of
internal energy
Calorimetry
A measure of the amount
of heat released or absorbed
in a chemical reaction.
q = mc∆T
Organic Chemistry
Functional Groups
Change of function, due to
the change in structure
Alcohol
Controlled Oxidation
Dehydration
Ether
Tertiary Alcohol
Secondary Alcohol
Ketone
Primary Alcohol
Aldehyde
Carboxylic Acid
Ester
Adding Water (H2O)
to the molecule
Hydrogenation
Adding a Hydrogen (H2)
to the molecule
Hydro Halogenation
Alkyl Halide
Adding a halogen to
the molecule
Geometric Isomers
Trans
Functional groups on the
opposite side of the C chain
Cis
Functional groups on the
same side of the C chain
Markovnikov's Rule
Rich get richer
Organic Molecules
Hydrogen and Carbon
Alkyne
At least 1 triple
bond
Most amount of tension
Methyne
Alkene
At least 1 double
bond
Methene
Alkane
Only single bonds
Methane
Least amount of tension
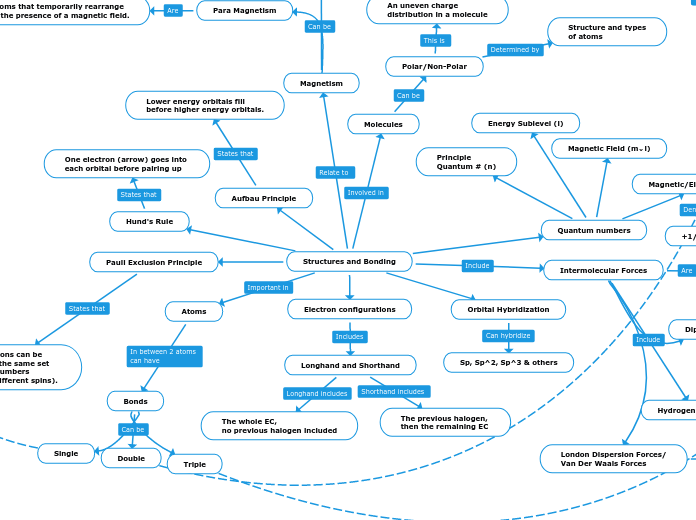
Structures and Bonding
Magnetism
Ferro Magnetism
Certain materials form to
be permanent magnets.
Iron, Cobalt, Nickel
Para Magnetism
Atoms that temporarily rearrange
in the presence of a magnetic field.
Intermolecular Forces
London Dispersion Forces/
Van Der Waals Forces
Temporary (short-term) forces
that can occur between any two molecules
Polar or non-polar
Hydrogen Bonding
A type of dipole-dipole
specific to hydrogens
Dipole-Dipole
Magnetic attraction between
S^+ and S^-
Forces of attraction that appear
between two or more molecules
Molecules
Polar/Non-Polar
Structure and types
of atoms
An uneven charge
distribution in a molecule
Atoms
Bonds
Triple
Double
Single
Orbital Hybridization
Sp, Sp^2, Sp^3 & others
Pauli Exclusion Principle
No two electrons can be
identified by the same set
of quantum numbers
(must have different spins).
Hund's Rule
One electron (arrow) goes into
each orbital before pairing up
Aufbau Principle
Lower energy orbitals fill
before higher energy orbitals.
Quantum numbers
Energy Sublevel (l)
Magnetic/Electron Spin (m⌄s)
+1/2 or -1/2
Magnetic Field (m⌄l)
Principle
Quantum # (n)
Electron configurations
Longhand and Shorthand
The whole EC,
no previous halogen included
The previous halogen,
then the remaining EC