por Wilson Taysia hace 5 años
814
Chemical nomenclature
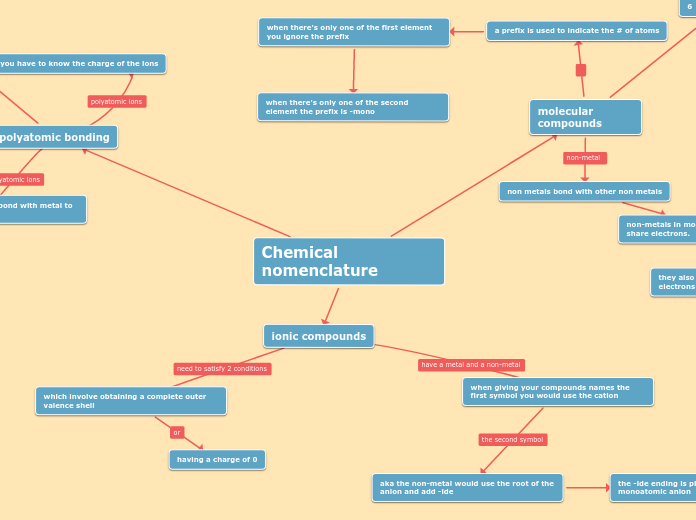
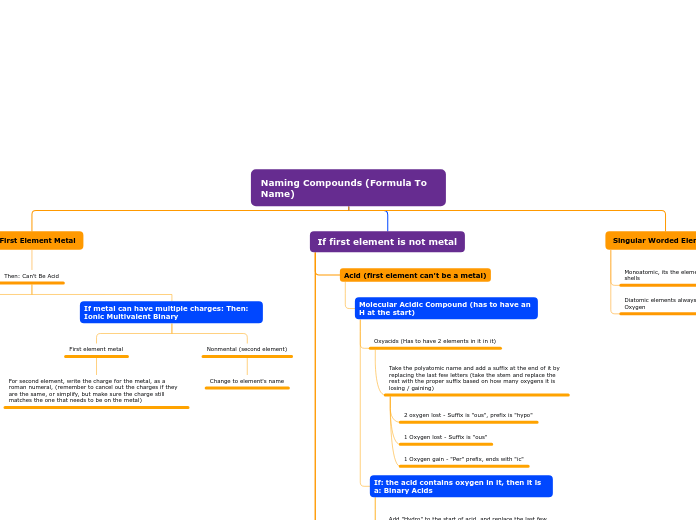
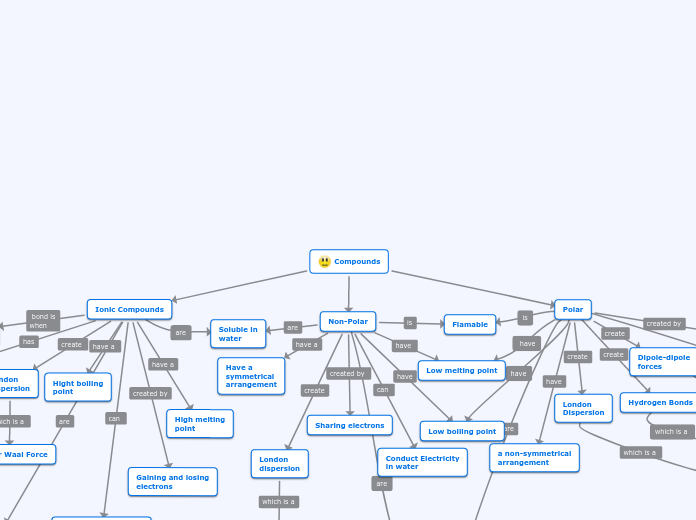
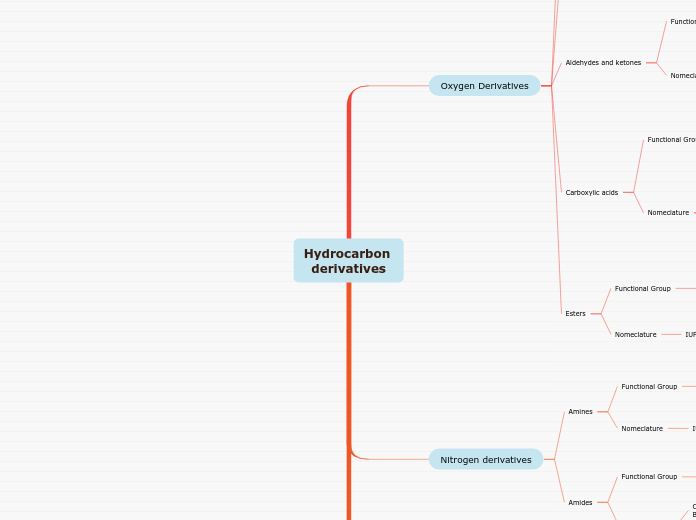
Chemical nomenclature encompasses the systematic naming of compounds based on their composition and structure. In polyatomic bonding, particularly with radicals and metals, ionic compounds are formed, often involving polyatomic anions that include oxygen.