I have clearly showed all 3 ways to convert from one unit to another eiether by using molar mass and moles to calcualte mass, and using other terms to calcualte each other. I have also drawn diagram to represent this relationship
s
I converted one concentration unit (parts per million) to another oncentration unit (parts perbillion)
#3 Students will conduct an inquiry to calculate the percentage composition of a compound
and understand the law of definite proportions. [TI]
I can analyze the data obtained during the experiment and make conclusions. 3.3
I can conduct an experiment to find the percent composition of a compound. 3.2
I can explain the law of definite proportions. 3.1
#4 Students will explain the relationship between the empirical formula and the molecular formula of a chemical compound and solve problems based on it. [C and TI]
I can calculate the empirical and molecular formulas of compounds based on the percent composition data. 4.2
I can explain the difference between empirical and molecular formulas.
4.1
file:///C:/Users/Owner/Downloads/PART%20B1_%20Individual%20Research.pdf
file:///C:/Users/Owner/Downloads/Indivisual%20Part%20A2%20(%20Recognizing%20Air%20Pollution%20and%20it's%20impacts)%20(1).pdf
file:///C:/Users/Owner/Downloads/Indivisual%20Part%20A1%20(Reflecting%20on%20your%20Carbon%20Footprint).pdf
This was an reserch part of the first assignment from the video this also describes the impact of that pollination has on the air quality in sarnia and why putting so many faiclities and posionous companies have riuned the air quaility of that area by alot.
fOR THIS i WROTE THE CHEMICAL EQUATIONS BASED ON THE GIVEN DESCRIPTION OF THE CHEMICAL EUQATION
Clearly I showed if the reaction was going to form a preicpiate or not by putting S or aq from the solubility chart which predicted the products of the double displacement reaction
i CLEARLY WROTE WHICH TYPE OF REACTION IT WAS ON THRE RIGHT SIDE
file:///C:/Users/Owner/Downloads/Indivisual%20Part%20A2%20(%20Recognizing%20Air%20Pollution%20and%20it's%20impacts).pdf
I was able to make a video about the ontarian government and the argiculture problem . Remember I introduced doug ford as special guest. Then I also did google slides presentation for the common chemcials assignment. Lastly, I also shown oral means of communication as I WROTE A PRAGRAPGH ONTHE DIFFERENT CHEMICAL FACILITIES THAT ENDANGER poc IN CANADA/
iN THIS EVIDENCE i SHOWED THE GRASS METHOD OF ORANGISING SHOWING ALL MY STEPS
Here you can see the process of grass and how I EFFECTIVLY USED IT TO ANSWER THE QUESTIONS. tHEN THE 2ND EVIDENCE SHOWS HOW i AGAIN USED GRASS (given required, analzysis, solution, and scetence). The next one after that shows grapghical relationship of the different laws in unit 5, and the last peiace of evidence is a chart filled out for the cohemical reactions lab showing I can easily organize and arrange my work in a table.
I have clearly named many covaLENT COMPOUNDs epsically in the types of reactions test you can see I have many covalent compounds. I also have a grapghic organizer explaining how to name and write them on the top . I also have my chemsitry vrieivew sheet for naming compounds and it explains how to name it very well aswell.
I have clearly shown the different moelculer strucutres using electron dot gfiragram along with showing the lewis strcutures of various compounds who are ionic of covalent
Clearly I have used terms such as electron affanity, ionization energy and other terms to answer questions that were on a unit test ofr sorry quest for unit 1
I can communicate my knowledge using a variety of means (oral, written, electronic presentations, videos etc.) (5.3)
All these are multpiule evidence of my understnading of istopes and radiosotopes along with subatomic particles in the worksheet given by you. Furthermore, I went as far as creating a venn diagram to show similatieries between them showing deep understanding
This table shows the relationships between the amount of ionization energy value and the corespsonding bond type effectivly explaining their deep relationship with each other |(this is also one of my grpaghic organizers along with the venn diagrams
#1 Student wills demonstrate an understanding of the periodic table and its trends [Ku,Ti]
I can analyze data pertaining to elements in the periodic table and identify trends. (1.3)
I can describe and explain general trends in the periodic table using electron arrangement and nuclear charge (e.g. ionization energy, electron affinity, electronegativity and atomic radius). (1.2)
https://docs.google.com/document/d/1_JPMnHru63wW3MSQFEvLqExOw5iQuxnw/edit?rtpof=true
This was the indivisual gropu assignment and I have clrearly desmonsrates great understnading of the terms as I have shown you differewnt graphs of the trends and answered questions based off of their coinciding reltionships between each other
I can name and describe the subatomic particles, explain the difference between isotopes and radioisotopes and discuss their use/impacts on society and the environment (1.1)
SCH3U Final Summative Concept Map
Main topic
Gases and atmospheric chemistry
#3 solve quantitative problems by performing
calculations based on Boyle’s law, Charles’s
law, Gay-Lussac’s law, the combined gas law,
Dalton’s law of partial pressures, and the ideal
gas law [AI]
I can solve problems involving the ideal gas law (3.4)
I can apply Dalton’s law of partial pressures (3.3)
I can solve problems involving the combined gas law (3.2)
I can solve problems involving Boyle’s law, Charles’s law, Gay-Lussac’s law, (3.1)
#2 determine, through inquiry, the quantitative
and graphical relationships between the pressure,
volume, and temperature of a gas [PR, AI]
I can graphically show the relationship between the pressure, volume, and temperature of a gas
I can describe the relationship (direct or inverse) between temp & volume, temp & pressure and volume & pressure (2.1)
#1 use appropriate terminology related to gasses
and atmospheric chemistry, including, but not
limited to: standard temperature, standard pressure,
molar volume, and ideal gas [C]
describe the different states of matter, and
explain their differences in terms of the forces
between atoms, molecules, and ions
I can use the kinetic molecular theory to explain the properties and behaviour of gasses in terms of types and degrees of molecular motion (1.3)
I can describe the difference between an ideal gas and real gas (1.2)
I can define STP and SATP, molar volume, ideal gas constant (1.1)
Solutions and solubility
#5 Students will use stoichiometry to solve problems involving solutions and solubility [TI]
I can use stoichiometry flow charts to solve problems related to solutions and solubility. 5.1
#4 Students will identify, using a solubility table, the formation of precipitates in aqueous solutions and write balanced total and net ionic equations to represent precipitation and neutralization reactions [C and TI]
I can write balanced total and net ionic equations
4.3
I can predict the formation of a precipitate using the solubility chart. 4.2
I can write balanced double displacement reactions that involve formation of a precipitate. 4.1
#3 solve problems related to the concentration of solutions by performing calculations involving moles, and express the results in various units (e.g., moles per liter, grams per 100 mL, parts per mill
I can convert one concentration unit to another concentration unit
I can express the solution concentration in appropriate units. 3.2
I can use appropriate concentration formulas to solve word problems related to solution concentration.
3.1
#2 students will describe the properties of water and use IMF’s to explain them. They should be able to predict and explain the behaviour of ionic, polar and nonpolar covalent substances in water. [KU and TI]
I can use IMF’s to predict and explain the behaviour of ionic, polar and nonpolar covalent compounds in water. 2.2
I can describe the properties of water using hydrogen bonding. 2.1
#1 Students will understand the meaning and use appropriate terminology related to aqueous solutions and solubility, including, but not limited to: solute, solvent, concentration, solubility, precipitate, ionization, dissociation, pH, dilute, solute, and solvent [C]
I can identify and use the impact of different factors on solubility of a substance in water.
1.3
I can use them appropriately where required. 1.2
I can explain the meaning of different terms used in this unit. 1.1
Quantities in chemical reactions
#4 Students will analyze on the basis of research, chemical reactions used in various industrial processes that can have an impact on the health and safety of local populations. [App.]
I can assess the effectiveness of some applications of chemical reactions that are used to address social and environmental needs and problems. 4.2
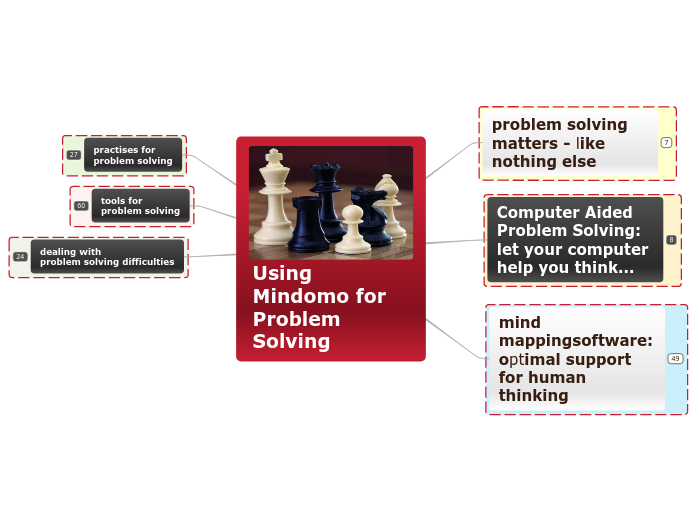
Here was the common chemcial assignment and I dsecribed how nitrous oxide is useful for making whip cream cans, for uses in healthcare idnsutry for oxgyen tanks, and also how it's used in toothpaste to make them chemicals eat away at the harmful bacteria that may expire the toothpaste faster
I can research and analyze the chemical reactions used in various industrial processes that can have an impact on the health and safety of local populations. 4.1
https://www.youtube.com/watch?v=moGSrv8MXks
In this video I described the impacts that agricultural farming could have showing all the munure going and polluting our rivers through the process of precipitation and boosting algal blooms destroying lake depdent bussinesses such as suhshi stores or seafood markes.
#3 Students will investigate different reactions by testing the products of each reaction [TI]
I can plan and conduct an inquiry to demonstrate different types of reactions. 3.1
I can carry out tests for products such as gasses (oxyge n, hydrogen and carbon dioxide 3.2
#2 Students will write balanced chemical equations to represent different types of reactions using the IUPAC nomenclature system [C]
I can include the states of reactants and products in the equation. 2.3
I can write formula equations based on the given description. 2.2
I can write word equations based on the given description. 2.1
#1 Students will demonstrate an understanding of the periodic table and its trends. [KU, TI]
I can explain the difference between a complete and incomplete combustion reaction. 1.5
I can use the solubility chart to predict the products of a double displacement reaction. 1.4
I can use the activity series to predict the products of a single displacement reaction. 1.3
I can predict the products of chemical reactions based on the type of reactions. 1.2
Subtopic
I can identify different types of reactions from the given description or given chemical equations. 1.1
Chemical reactions
#5 Students will explain the quantitative relationships in a balanced chemical equation and solve problems based on percentage yield and limiting reagents. [TI]
I can solve word problems related to percent yield and describe factors that impact the yield.
I can solve word problems involving stoichiometry, limiting and excess reagents
5.1
#6 Students will analyze and assess processes that involve the use of chemical quantities and calculations and understand their importance in real life. [App.
I can assess the importance of quantatiatve accuracy in real life applications and their impact on the envrionment if accuracy is not observed. 6.2
I can analyze the processes in the home, workplace and the environment that involve chemical quantities and calculations.
6.1
This describes real life explanations of stochiometry that involves quantities and calculations. Although basic, it still shows how we still need to use stochiometry to meet our daily needs in our everyday life.
#2 students will describe the relationships and solve problems between Avogadro’s number, the mole concept, and the molar mass of any given substance. [KU and TI]
I can solve word problems based on these formulas.
2.2
I can use the appropriate formula to isolate the unknown quantity in Nx = n x NA and n = m/M
2.1
#1 Students will understand and use appropriate terminology related to quantities in chemical reactions, including, but
not limited to: stoichiometry, percentage yield, molar mass
limiting reagent, mole, and atomic mass [C]
I can express each quantity using appropriate units.1,.2
I can explain the meaning of each term - moles, molar mass, percent composition, limiting reagent, excess reagent and percent yield. 1.1
MATTER TRENDS AND BONDING
#5 Students will properly communicate their knowledge, research, and/or investigation planning, results, and analysis.
I can properly analyze quantitative data by including any formulae utilized, showing all steps in problem solving, and/or using proper SI units. (5.4)
I can concisely present information in appropriate formats (charts, graphs, tables, etc.), using appropriate terminology (5.1)
I can properly cite and reference a variety of appropriate sources using APA formatting (5.2)
#4 Students will identify societal, safety and/or environmental issues associated with a common chemical. [App.]
I can explain the environmental impacts of using that substance, and how they can be mitigated. (4.2)
I can research and analyze the structure and properties of a commonly used, but potentially harmful chemical substance (e.g. a pesticide, fertilizer, household cleaning product, material from electronic device/battery, etc.). (4.1)
https://docs.google.com/presentation/d/1NzwOuuhMkD3Z8Nm1QrFub0Qq8-j5zuFIEwQN4Ugh6SM/edit?pli=1#slide=id.p
In this commonly chemcials used assignment I easily described the many chemical compounds and properties such as low metling point due to covalentbond as they can easily be broken as the don;ty only have intermoleculer forces that are joining them together. rather they share there electrons. I also explained the impact on the envrionemtn and how nitrous oxide can easily contaminate near by lakes with process of acid rain.
#3 Students will identify, name and provide chemical formulae for a variety of different compounds. [Comm.]
I can name and write the chemical formulae for binary and oxyacids (including oxy acid variants), and hydrates. (3.3)
I can name and write the chemical formulae for covalent compounds. (3.2)
I can name and write the chemical formulae for binary ionic, polyatomic & variant compounds (including those with multiple valences). (3.1)
#2 Students will explain the structure, formation, and properties of ionic and covalent compounds. [Comm.]
I can draw Lewis structures & build models to represent molecules. (2.3)
I can use electronegativity values to predict bond type (i.e. ionic, polar covalent and nonpolar covalent). (2.2)
I can use appropriate terminology related to chemical bonding. (2.1)