ZIDOVUDINE
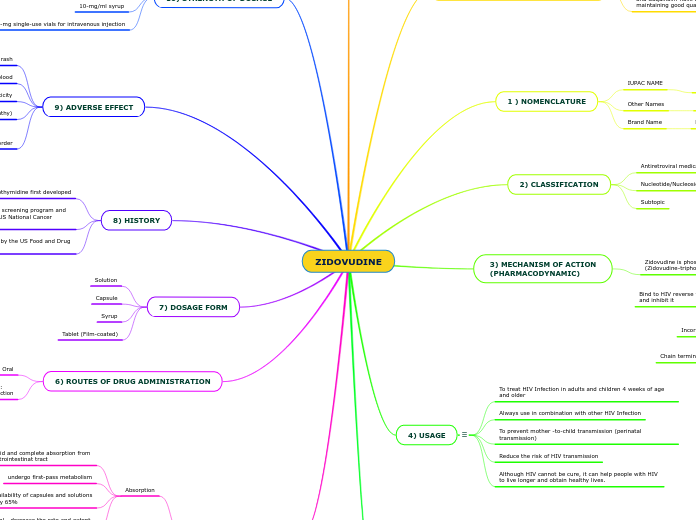
5) PHARMACOKINETICS
Elimination
Elimination will occur in the form of GZDV.For intravenous dosing, about 29% of the dose was excreted in the urine unchanged and about 45% of the dose was excreted as GZDV.
Metabolism
It metabolized by glucuronide conjugation to inactive metabolite, 3′-azido-3′-deoxy-5′- O-beta-D-glucopyranuronosylthymidine (GZDV).
Distribution
Volume of distribution via Intravenous Administration is 1.6± 0.6 L/kg
Absorption
Administration of high fat meal - decrease the rate and extent
of absorption
systemic bioavailability of capsules and solutions
is approximately 65%
undergo first-pass metabolism
Rapid and complete absorption from
gastrointestinat tract
6) ROUTES OF DRUG ADMINISTRATION
Parenteral Route :
Intravenous Injection
Oral
7) DOSAGE FORM
Tablet (Film-coated)
Syrup
Capsule
Solution
8) HISTORY
March 1987 : Approval of zidovudine by the US Food and Drug Administration
1983 : Burroughs Wellcome began a screening program and do further test that contracted with US National Cancer Institute (NCI)
1964 : Azidothymidine first developed
9) ADVERSE EFFECT
Blood disorder
Neutropenia (reduced numbers of white blood cells )
Anemia (reduced numbers of red blood cells )
Muscle weakness (Myopathy)
Hepatotoxicity
Build up of lactic acid in blood
Hypersensitivity reaction or rash
10) STRENGTH OF DOSAGE
20-ml/200-mg single-use vials for intravenous injection
10-mg/ml syrup
100-mg capsules
300-mg tablets
References
Webmd.com. n.d. Zidovudine.(July 2020) Available at: https://www.webmd.com/drugs/2/drug-4386/zidovudine-oral/details#:~:text=Some%20products%20that%20may%20interact,NSAIDs%20such%20as%20ibuprofen%20or [Accessed 5 July 2020].
Drugbank.ca. 2020. Zidovudine - Drugbank. [online] Available at: https://www.drugbank.ca/drugs/DB00495#BE0004136l
Niaid.nih.gov. 2018. Antiretroviral Drug Discovery And Development. [online] Available at: https://www.niaid.nih.gov/diseases-conditions/antiretroviral-drug-development
Zidovudine Dosage, Side Effects. (2020, January 20). AIDSinfo. Derived from https://aidsinfo.nih.gov/drugs/4/zidovudine/0/patient#:%7E:text=Some%20side%20effects%20of%20zidovudine,as%20severe%20anemia%20or%20neutropenia.
4) USAGE
vh
Although HIV cannot be cure, it can help people with HIV
to live longer and obtain healthy lives.
Reduce the risk of HIV transmission
To prevent mother -to-child transmission (perinatal transmission)
Always use in combination with other HIV Infection
To treat HIV Infection in adults and children 4 weeks of age and older
3) MECHANISM OF ACTION
(PHARMACODYNAMIC)
Zidovudine is phosphorylated to active metabolites
(Zidovudine-triphosphate)
Bind to HIV reverse transcriptase enzyme competitively
and inhibit it
Incorporate into viral DNA
Chain termination of DNA synthesis occur
2) CLASSIFICATION
Subtopic
Nucleotide/Nucleoside Reverse Transcriptase Inhibitors
Antiretroviral medication
1 ) NOMENCLATURE
Brand Name
Retrovir
Other Names
ADZ ,ZDV , Azidothymidine
IUPAC NAME
1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
12)DRUG AVAILABILITY IN MALAYSIA
Combination of zidovudine with new drugs such as Lamivudine
and Saquinavir have been shown to be more effective in maintaining good quality of life in AIDS patients
Easily available in Malaysia
11) DRUG-DRUG INTERACTION
(Drug that may interact with
Zidovudine)
Ganciclovir, Dapsone, Trimethoprim
May suppress bone marrow function
Ibuprofen , Naproxen
Drug interaction may
affect the kidney
Probenecid
Orlistat
HIV medication become less effective
Interfere the drug absorption of HIV medication