jonka Osalumese Ikpotokin 2 kuukautta sitten
45
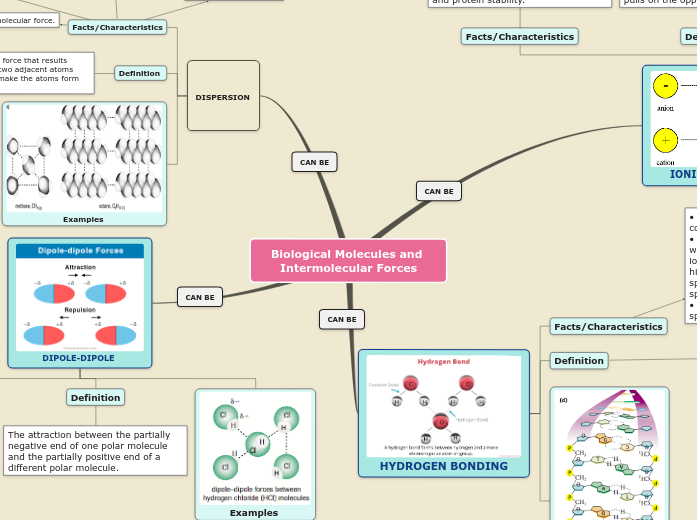
Biological Molecules and Intermolecular Forces
Intermolecular forces are crucial in understanding the behavior and properties of molecules. Dispersion forces, also known as London forces, are temporary attractive forces that occur when electrons in adjacent atoms form temporary dipoles.