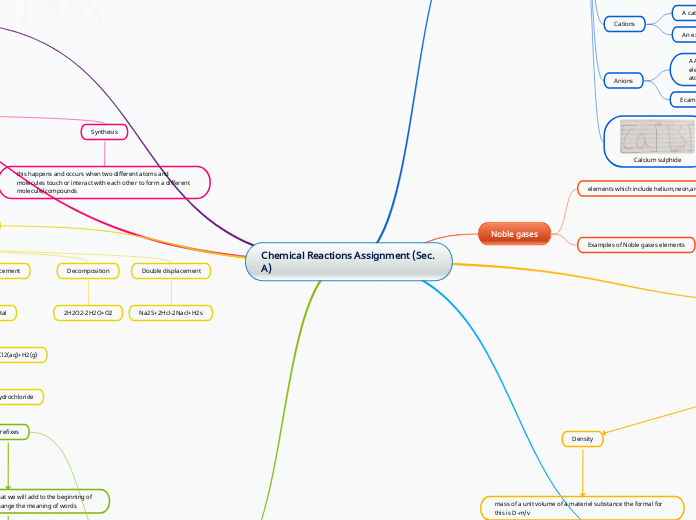
Chemical Reactions Assignment (Sec. A)
Nomenclature
Usage of roman Numerals
The roman numerals are used when writing equations or formulas. it represents the charge and the transition metal ions. For example Fe2+ as well as Fe3+ will be used to distinguish the difference. Fe2+ will be named iron (ll) and Fe3+ will be known as Iron (lll)
Prefixes
A prefix is a letter or word that we will add to the beginning of a word. by doing this it will change the meaning of words
Some examples of prefixes would be mono=1 di=2 tri=3 tetra=4 penta=5 etc
Chemical reactions
Double displacement
Na2S+2Hcl-2Nacl+H2s
2H2O2-2H2O+O2
zinc metal
Zn(s)+2Hcl (aq)-znCl2(aq)+H2(g)
zinc reacts with hydrochloride
2H2+O2-2H2O
Methane
Chemical equation for Methane
Ch4+2O2-CO2+2H2O
Balanced chemical equations
Types of Balanced chemical equations
Synthesis
this happens and occurs when two different atoms and molecules touch or interact with each other to form a different molecule/compounds
double displacement
This occurs when parts of a ionic compound are being exchanged and traded which will make two brand new compounds
Single displacement
This happens when a element will replace and kick out another element inside of a compound. metals will only replace metals and non metals will only replace nonmetals
Combustion
This equation will occur when a substance of some sort will react with the oxygen to end up and release energy. When methane burns into oxygen it will release carbon dioxide
Decomposition
A decomposition will happen when one reactant happens to break and separate into two or more products. The way we represent this is AB-A+B
Same number of each atom on both sides of the equation
Total charge is also the exact same
Covalent Compounds
H20
Water
Oxygen
Oxygen is a chemical element which only contains one type of atom. Its atomic number is eight which means also 8 protons in the nucleus
O2
H2
Hydrogen
Hydrogen is a chemical element with the atomic number 1. it is the lightest element. Most of hydrogen exist in water and organic compounds
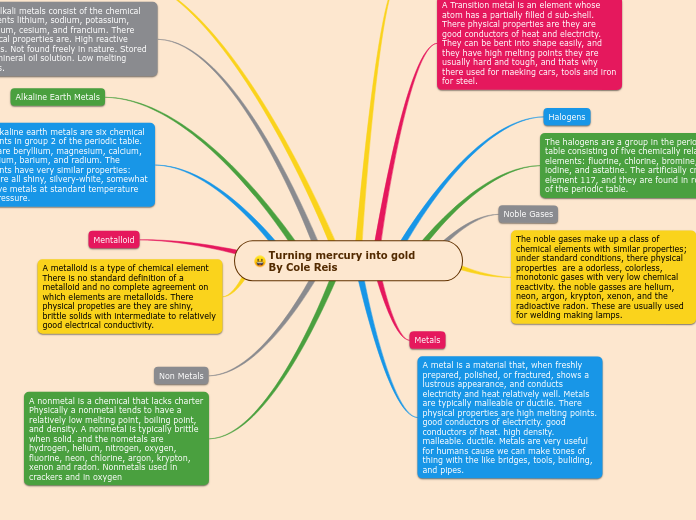
Metals and Non Metals
Examples of some non metals
Chlorine
sulfur
carbon
oxygen
properties of metals
shiny
they are very hard
Most elements you see on the periodic table are considered metals.
Examples of different elements on the periodic table
Iron
copper
silver
zinc
gold
Properties and bases
bases
Sodium hydroxide
(NaOH)
Barium hydroxide
(ba(OH)2)
Calcium Hydroxide
(Ca(OH)2)
properties
Boiling point
the boiling point of a liquid is when the temperature reaches to a point where it begins to chane into steem/ vapour substances
Melthing point
The temperature at what the solid or liquid from a pure substance to then be known as an equilibrium
Density
mass of a unit volume of a materiel substance the formal for this is D-m/v
Noble gases
Examples of Noble gases elements
organesson (og)
xenon(xe)
Krypton (Kr)
Helium (He)
Neon(Ne)
Radon(rn )
elements which include helium,neon,argun
Ionic compounds
Calcium sulphide
Chemical formula
CaS
Anions
Ecamples of Anions are Iodide,chlorine and Hydroxide
A Anion is a ion with a negative charge meaning it has more electrons than protons. These are formed when one or more atom gains electrons
Cations
An examples of Cation is Calcium,Potassium and Hydrogen
A cation is a positively charged ion
Ionic compounds are neutral compounds made up of positively charged ions which are known to be called cations and negatively charged ions are known as anions
Lithium Fluoride
Inorganic compound. this is a colourless solid which transforms or transitions into a white decreasing crystal sizes
Chemical name- LiF
Subtopic
Sodium fluoride
Sodium fluoride is made into toothpaste as sodium fluoride makes your teeth more decay and resistant to bacteria which will cause cavities
Chemical name-NAF (Na+)