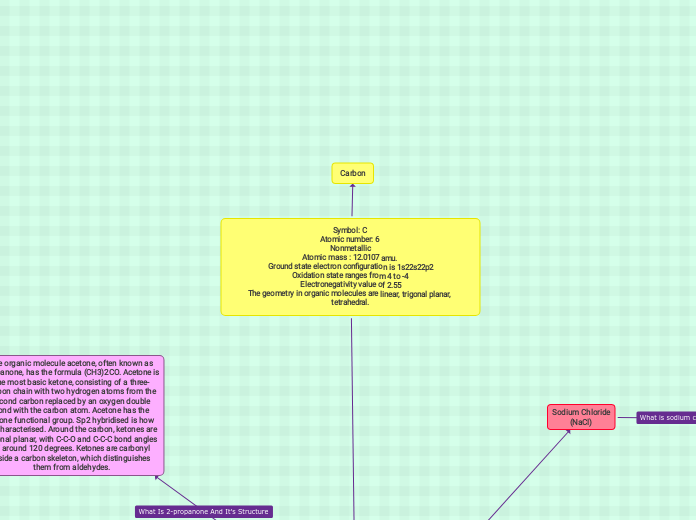
Symbol: C
Atomic number: 6
Nonmetallic
Atomic mass : 12.0107 amu.
Ground state electron configuration is 1s22s22p2
Oxidation state ranges from 4 to -4
Electronegativity value of 2.55
The geometry in organic molecules are linear, trigonal planar, tetrahedral.
Carbon
Carbon
Containing Molecules
2-propanone
(C3H6O)
(acetone)
The organic molecule acetone, often known as propanone, has the formula (CH3)2CO. Acetone is the most basic ketone, consisting of a three-carbon chain with two hydrogen atoms from the second carbon replaced by an oxygen double bond with the carbon atom. Acetone has the ketone functional group. Sp2 hybridised is how it's characterised. Around the carbon, ketones are trigonal planar, with C-C-O and C-C-C bond angles of around 120 degrees. Ketones are carbonyl inside a carbon skeleton, which distinguishes them from aldehydes.
Acetone is a breakdown product of animal fat metabolism that can be found in plants, trees, forest fires, and vehicle exhaust. The amount of acetone released into the environment by industrial activities is higher than that released by natural processes. In high doses, acetone is poisonous. (NCI04). Acetone is an organic solvent that is colourless, volatile, and flammable It is combustible, readily evaporates, and dissolves in water. Other substances can also be dissolved with it.
Acetone is used to create plastic, textiles, pharmaceuticals, and other products. Paint and nail polish removers use it as a solvent.
Acetone molecules contain a polar carbonyl group on their surface that allows them to accept hydrogen bonds from other substances. Each hydrogen atom's slightly positive charge can attract slightly negative oxygen atoms on neighbouring water molecules, establishing hydrogen bonds. Which is why As a result, acetone is soluble. Acetone is extremely flammable because it is a gaseous hydrocarbon, which means that the bonds between individual molecules are weak, and the bonds between atoms are also weak. As a result, when heat is applied, there is no need to disrupt intermolecular bonds, and atom-atom bonds are simply broken. Acetone is very flammable and swiftly decomposes. The phase transition from a liquid to a gas, as is customary, necessitates the use of heat energy. When acetone transitions from a liquid to a gas, it removes heat energy from whatever comes into contact with it, resulting in a chilly sensation.
Acetone evaporates quickly, and while it is made naturally, it is made commercially by manually joining three carbon atoms, six hydrogen atoms, and one oxygen atom to form the compound element (CH3)2CO.
To name acetone, we must first begin with counting the length of the carbon chain. Since there is 3 carbons present, the correct prefix that we will use is -prop. Second we will locate the position of the oxgyen of which number of carbon it is located. It is located in the middle of the carbon chain which is carbon 2. Therefore, the 2- will come before prop-. Lastly, the suffix
-ane is replaced with -anone to name ketones. Therfore, the suffix for acetone will be -anone.
Following these rules, the nomenclature will result in 2-propanone.
Complete combustion of 1.00 mol of acetone (C3H6O) liberates 1790 kJ:
C3H6O(ℓ) + 4O2(g) ---> 3CO2(g) + 3H2O(ℓ)
ΔH combo = −1790 kJ
The values below is what we will use to calculate the enthalpy of formation of acetone.
ΔHf, O2 = 0
ΔHf, CO2 = −393.5
ΔHf, H2O = −285.83
Solution
1) Hess' Law:
ΔHrxn = Σ ΔHf, products − Σ ΔHf, reactants
2) Substitute values into equation:
−1790 = [ 3 (−393.5) + 3 (−285.83) ] − [ (ΔHf, acetone ) + (4) (0) ]
−1790 = −2037.99 − ΔH°f, acetone
247.99 = −ΔHf, acetone
ΔHf, acetone = −247.99 kJ/mol
To three sig figs, the value is −248 kJ/mol.
The heat of formation of acetone is 248kJ/mol.
Dipole-dipole forces will be present since acetone possesses a dipole. Isobutyl alcohol, like water, has a dipole and may hydrogen bind. Since hydrogen bonding attracts water molecules to one another, it evaporates slowly. Although acetone does not engage in hydrogen bonding, its intermolecular forces are less, therefore it evaporates the fastest.
Breathing acetone at moderate to high concentrations for a short time might irritate your nose, throat, lungs, and eyes. Headaches, dizziness, confusion, a quicker pulse, nausea, vomiting, blood effects, passing out and potential coma, and a shorter menstrual cycle in women are all possible side effects.
Step 1: Find the compound's longest carbon chain.
Step 2: Identify the carbon chain with the longest length.
Step 3: Determine what the suffix (ending) should be.
Step 4: Count the number of carbon atoms in your body.
Step 5: Give the side groupings a name.
Step 6: Arrange the side groupings alphabetically.
Sucrose
(C₁₂H₂₂O₁₁)
Sucrose is a disaccharide
Sucrose is a disaccharide which means that is a molecules made up of two monosaccharides. Sucrose's monomers are a glucose molecule and a fructose molecule bonded together.
Sucrose is used as a sweetener in meals and soft drinks, in syrup production, confectionery, preserves and jams, demulcents, medical items, and caramel. Sucrose also serves as a chemical carrier for detergents, emulsifiers, and other saccharose derivatives. Table sugar is refined from sucrose, which is naturally generated by plants. Sucrose is also the most prevalent carbohydrate used by plants to transfer carbon.
Sucrose is harvested and processed from either sugarcane or sugar beet for human use. Sugar mills smash the cane and generate raw sugar, which is then delivered to refiners to be refined into pure sucrose. Sugar beet factories refine sugar straight from the beets. The raw sugar crystals are washed before being dissolved in a sugar syrup, which is then filtered before being run over charcoal to remove any remaining colour. The sugar syrup is then concentrated by boiling under vacuum and crystallised as the last purification step, yielding transparent, odourless, and delicious crystals of pure sucrose.
The molar Mass is 342.30 g/mol. The density of sucrose is 1.587 g/cm3. Sucrose is a molecular solid. Molecular solids are held together by intermolecular forces (IMFs). Sucrose has hydrogen bonding, dipole-dipole and London dispersion forces. Sucrose is a polar molecule and has covalent bonds. The melting point of sucrose is 185.85 degrees celsius (459K).
The negative and positive regions on the polar sucrose molecules are attracted by the polar water molecules, causing sucrose to dissolve in water. In most cases, electron sharing is uneven, thus covalently bound molecules have some polarity. Polar interactions are common in organic compounds, but they are typically weak. As a result, sucrose possesses strong covalent bonds that keep a single sucrose molecule together, but weak polar bonds that hold nearby sucrose molecules together. Ions are formed when sodium chloride dissolves in water. The ions that are being charged have the ability to conduct electricity. Sucrose is a covalent molecule that does not ionise in water and so does not create electrically conducting ions.
The physical appearance of sucrose is white, crystalline and a solid.
SUcrose contains a hydroxyl group, an acetal group, and a glycosidic bond. Sucrose monomers (glucose and fructose) are joined together via glycosidic linkage.
The English scientist William Miller invented the term sucrose in 1857, combining the French sucre ("sugar") and the usual chemical suffix for sugars, -ose. In scientific literature, the shortened word "Suc" is frequently used for sucrose.
11mol H2O = -285.8KJ/mol
12CO2 = -393.5KJ/mol
deltaHf= [(11mol x -285.8KJ/mol) + (12mol x -393.5KJ/mol)] + [5639.7KJ/mol]
= (-3143.8 + -4722) + 5639.7
= -7865.8 + 5639.7
= -2226.1
Therefore, the heat of formation of sucrose is -2226.1KJ
Polyethene
(polyethylene)
(C₂H₄)ₙ
Polyethylene (PE) is a light, versatile synthetic resin made from the polymerization of ethylene. Polyethylene is a common type of thermoplastic and is a homopolymer.
A homopolymer is a polymer that is made of only one type of monomer.
Polyethylene's monomer is ethene.
Monomers are small molecules with functional groups which allows them to form polymers.
It is used for many products today, such as packaging film, trash/grocery bags, agricultural mulch, wire and cable insulation, squeeze bottles, toys, and housewares.
Polyethylene has a strong force because of the long repeating units of ethene monomers. There are many types of plastics including polymers that have ethene monomers. Thermoplastic polymers is a synthetic substance that can be molded when heated and retains it shape when cooled. They do not contain many cross-links which makes them easier to soften at high temperatures.
The density of polyethene polymers is classified. There are many various density types but the two most common ones are low-density polyethene (LDPE) and high-density polyethene (HDPE). LDPE is created by combining two or more double-bonding chemicals in a tiny volume.
Low density polyethylene (LDPE) is soft, more flexible than HDPE, and lightweight plastic. Low-temperature flexibility, toughness, and corrosion resistance are all characteristics of LDPE. It is not suitable for applications requiring rigidity, high temperature resistance, or structural strength.
In comparison to HDPE, the polymer chains of LDPE are extremely branched. This branching inhibits the chains from neatly stacking next to one other, lowering the intermolecular attraction forces. As a result, the plastic is softer and more flexible, but its tensile strength is reduced.
Containers, dispensing bottles, wash bottles, tubing, plastic bags for computer components, and different moulded laboratory equipment are among the most common uses for Low-Density Polyethylene (LDPE).
.
CH3
|
CH2
|
CH2
|
CH2
|
-CH2-CH2-CH-CH2-CH2-CH-CH2-CH2-
|
CH2
|
CH3
HDPE is more stiff and robust, as well as more chemical resistant. It can resist higher temperatures than LDPE because of its higher melting point (135° C).
HDPE has a stronger crystalline structure than LDPE, making it more opaque, rigid, and with a higher tensile strength.
High-density polyethylene is used in crates, trays, milk and fruit juice bottles, food packaging caps, jerry cans, drums, and industrial bulk containers, among other uses. HDPE offers an acceptable impact strength to the finished product in such situations.
-CH2-CH2-CH2-CH2-CH2-CH2-CH2-
The name of the monomer (the'source') from which it is derived is used to call a homopolymer. In terms of polyethene, poly(ethene). The "poly" prefix is followed by the monomer, which is ethene. Monomers can be named according to IUPAC guidelines or by adopting well-known traditional names.
H H
| |
C-C
| |
H H
H H H H H H H H H H H H H H H H H H H H H H H H
| | | | | | | | | | | | | | | | | | | | | | | |
C=C C=C C=C C=C C=C C=C -> C-C-C-C-C-C-C-C-C-C-C-C
| | | | | | | | | | | | | | | | | | | | | | | |
H H H H H H H H H H H H H H H H H H H H H H H H
Ethene Monomers Polyethylene Polymers
n[CH2 = CH2] ----> [CH2 -CH2]n
"n" has large integral value
In this polymerization reaction, every molecule of ethylene involves breaking of one C = C (double bond) and formed two C-C (single bonds). The amount of energy which is required to break one mole of = 590 KJ. Thus, energy released in the formation of two moles of C-C single bond = 2 x 331 = 662 kJ Net energy released per mole of ethylene = 662- 590 = 72 kJ Enthalpy of polymerisation per mole of ethylene at 298 K (ΔH)= -72 kJ/mol.
Polymer chains are held together effectively because Van der Waals forces attract polymer molecules. Although these intermolecular interactions are weak on individually, polymer chains can have very long, thousands of carbon atoms, and the attractive forces add up.
H H H H
| | | |
C=C ---> --C--C--
| | | |
H H H H
Ethene Polyethylene
Monomer Monomer
Sodium Chloride
(NaCl)
Sodium chloride, often known as table salt, is one of the most well-known and frequently used compounds.
Table salt is the most common form of sodium chloride, which is widely used in the food sector for flavour and preservation. It's also used to make sodium hydroxide, sodium carbonate, baking soda, and hydrochloric acid, among other vital compounds. It's also used in oil refineries, textile mills, paper and pulp mills, fire retardants, rubber manufacturing, and road building. De-icing of roads and walkways in cold and snowy areas is another major use. Saline solutions are also employed in a variety of medicinal applications.
Each Na+ ion in solid NaCl is surrounded by six chloride ions in an octahedral geometry, giving it a crystalline structure.
The chemical formula for sodium chloride is NaCl, and its molar mass is 58.44g per mol. It's an ionic compound made up of the sodium cation (Na+) and the chloride anion (Cl-)
Table salt is made up of the elements sodium chloride and chloride. It's caused by the interaction between Na+ and Cl ions. Na + Cl2 --> NaCl. This is a synthesis reaction, as a result. Under the correct conditions, sodium metal and chlorine gas combine to make salt. The ionic substance NaCl is produced during this process. This is because it is a nonmetal (Na) and a metal bond (Cl). It's also vital to understand that electron transfer forms an ionic molecule. An electron is lost by sodium and gained by chlorine. Due to the electrostatic interaction between the particles, this reaction is extremely favorable. A lot of light and heat is emitted throughout the process. The salt that results is generally inert and stable. Unlike sodium and chlorine, which it is comprised of, it will not experience any explosive reactions. The balanced equation is 2Na(s)+Cl2(g) --> 2NaCl(s)
The saltiness of sea and ocean waters is due to the presence of sodium chloride. Sodium chloride makes up around 1-5 percent of saltwater. It can also be found in the form of the mineral halite, or rock salt.
Evaporation of sea water or salt water (brine) from salt lakes and brine wells is used to generate salt on a big scale. Since saltwater includes a variety of other salts (calcium, magnesium, and other elements), the evaporation process is carefully monitored to ensure that the various salts precipitate at different periods depending on their solubility. Mining the rock salt deposits is another important technique of production.
Most other liquids are insoluble or very slightly soluble in sodium chloride, which is highly soluble in water. It produces tiny, clear cubic crystals that range in hue from white to colourless. Although sodium chloride has no odour, it has a distinct flavour. It's an ionic composition with an equal amount of positively and negatively charged sodium and chloride ions. When sodium chloride is melted or dissolved in water, the ions are free to move about, making it a conductor of electricity. It may be broken down into sodium and chlorine by sending an electrical current through it. It is a solid that is stable. Only at high temperatures can it disintegrate, releasing deadly fumes of hydrochloric acid (HCl) and disodium oxide (Na2O).The melting point of sodium chloride is 801 degrees Celsius, which is very high. It is a white crystalline solid with a density of 2.16 g/mL and a density of 2.16 g/mL. It's also available as saline solutions, which are aqueous solutions with various concentrations.
When NaCl is dissolved in water, it splits into two components: Na+ and Cl. These ions are highly attracted to the water molecule's partial negative and partial positive ends. As a result, ion-dipole interactions are the most important intermolecular forces in the dissolution of NaCl in water.
In sodium chloride, the ions are arranged in a massive ionic framework (also known as a giant ionic lattice). A crystal is formed as a result of this orderly arrangement. In sodium chloride crystals, this pattern is replicated in all directions, resulting in a massive three-dimensional lattice structure. When salt is combined with water, the covalent bonds of water are stronger than the ionic bonds in the salt molecules, and the salt dissolves. The ionic bond that held the sodium and chloride ions together is broken as water molecules pull them apart. The sodium atoms and chlorine atoms separate when sodium chloride dissolves in water due to the effect of the water molecules. They're free to travel about as positively and negatively charged ions in the water. The solution can conduct electricity because of the charge separation. The positive and negative ions have strong electrostatic attractions that require a lot of heat energy to overcome. The melting and boiling points of all ionic compounds are quite high.
Diamond
(C)
Diamond is a form of carbon which contains massive of only carbon atom structures that are held together by covalent bonds.
Another solid form of carbon known as graphite is the chemically stable form of carbon at ambient temperature and pressure, although diamond transforms to it very slowly. It has a radically distinct formation mechanism and crystal structure. As a result, graphite is so soft that it can be used to write with, but diamond is so hard that it can only be scratched by another diamond.
Diamond is a massive covalent structure that consists of strong covalent bonds which connects each carbon atom to four other carbon atoms, forming a regular tetrahedral network structure with no free electrons. Diamond is the only mineral formed entirely of one element which is carbon. It usually contains 99.95 percent carbon. One or more trace elements, which are atoms that aren't part of the diamond's fundamental chemistry, can make up the remaining 0.05 percent.
The formula only consists of repeating units of carbon (C)
The melting point of diamond (almost 4000°C) is quite high. Before melting, the structure's extremely strong carbon-carbon covalent bonds must be broken throughout. The hardest substance is diamond. This is due to the requirement to break extremely strong three-dimensional covalent bonds. Diamond has an isometric crystal structure, which means the carbon atoms are bonded in the same way in all angles. Electricity does not conduct through diamond. All of the electrons are tethered to the atoms and are unable to move freely. Water and organic solvents do not dissolve diamond which makes it insoluble in water/organic solvents. There are no potential interactions between solvent molecules and carbon atoms that would surpass the covalently bonded carbon atoms' attractions.
Each carbon atom in a diamond is linked to four other carbon atoms, generating a massive covalent structure. There are no delocalized electrons in the structure, hence it does not conduct electricity. Since diamonds are comprised of carbon, they develop as carbon atoms at high temperatures and pressures, bonding together to form crystals. Each carbon atom participates in four of these incredibly strong covalent bonds that form between carbon atoms, which is why a diamond is such a hard substance. Water does not dissolve diamond. This is because there are no free electrons or ions in a diamond because each atom is connected to its neighbours by four strong covalent bonds. Diamond is extremely hard due to its three-dimensional arrangement of carbon atoms bonded together by strong covalent bonds. Because a considerable amount of energy is required to overcome the many strong covalent bonds, diamond has a relatively high melting point.
Since diamond is the hardest mineral in the world. Cutting equipment, such as diamond-tipped glass cutters and oil rig drills, benefit from this. Diamonds are used as jewelry. The diamond's crystalline, dazzling structure allows it to be broken into smaller structures and then engraved with a pattern. When combined with other metals such as platinum, silver, gold, titanium, tungsten, and others, it has a high amount of optical dispersion, making it both attractive and distinctive. Diamonds are also employed in the manufacture of automobiles. Every high-tech vehicle has 1.5 carats of diamonds.
Diamond makes four covalent connections, resulting in a tetrahedral structure with a bond angle of 109.5 degrees. Diamond has a very hard and have a high melting/boiling point because all of the outside electrons form a covalent link. As a result, the structure has no weak Van der Waals forces.