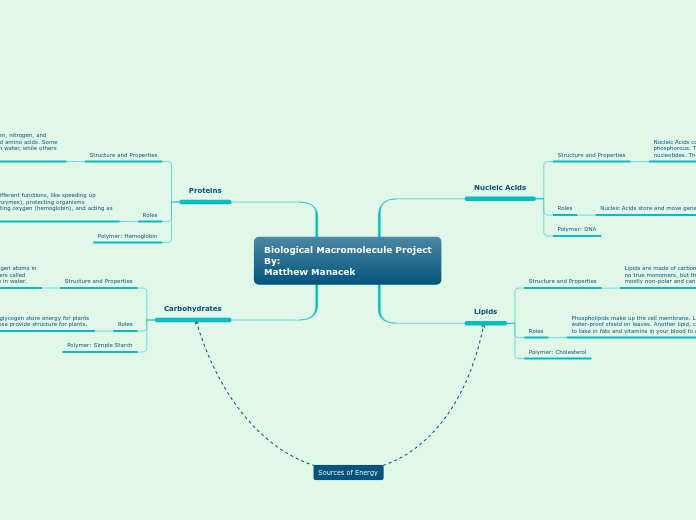
Biological Macromolecule Project
By:
Matthew Manacek
Carbohydrates
Polymer: Simple Starch
Carbohydrates like starch and glycogen store energy for plants and animals. Others like cellulose provide structure for plants.
They are a quick source of energy because they break apart into their monomers break apart easily upon digestion. They also provide a good rigid structure for plants.
Carbohydrates provide living organisms with the energy needed to complete activities. They are a great energy supply for people and other animals. They also give plants protection, so they can survive harsh environments.
Link to Real Life Situations:
A football player might eat a granola bar with carbohydrates in it to give himself energy before a game.
Carbohydrates contain carbon, hydrogen, and oxygen atoms in a 1:2:1 ratio. Carbohydrates are made of monomers called monosaccharides. They are polar and can dissolve in water.
Monosaccharide literally means "one sugar." An example of a monosaccharide is glucose. When glucose monomers are combined over and over again, they create polysaccharides like starch and cellulose
Proteins
Polymer: Hemoglobin
Proteins have many different functions, like speeding up chemical reactions (enzymes), protecting organisms (antibodies), transporting oxygen (hemoglobin), and acting as a hormone (insulin).
Enzymes speed up chemical reactions by reducing their activation energy. Antibodies are produced as a reaction to antigens entering the body, and when they return, antibodies deactivate them. Hemoglobin attaches to oxygen and releases it to body tissues. Insulin allows the cells in the muscles, fat and liver to absorb glucose that is in the blood, which serves as energy to these cells.
Enzymes speed up reactions in the body; without them, some bodily functions would take too long. Without hormones like insulin, our bodies wouldn't be able to develop properly. Antibodies protect our bodies from sickness. Without hemoglobin, oxygen would not get to the right parts of our bodies, and we would die.
Link to Real-Life Situations:
To get all the nutrients from the food we eat, we use enzymes, which speed up digestion. When we get sick, antibodies are produced to prevent us from getting sick the same way.
Proteins contain carbon, hydrogen, oxygen, nitrogen, and sulfur. They are made of monomers called amino acids. Some proteins are polar and can be dissolved in water, while others cannot, depending on their structures.
Amino acids are made of a carboxyl, amino, hydrogen, and R group around a central carbon. Differences in the R group produce different amino acids. Combining amino acids over and over again through dehydration synthesis creates proteins like enzymes.
Lipids
Polymer: Cholesterol
Phospholipids make up the cell membrane. Lipids make up the water-proof shield on leaves. Another lipid, cholesterol, helps to take in fats and vitamins in your blood to make hormones.
Lipids are not soluble in water, so they are perfect for making up the water-proof shield on leaves. Cholesterol is hydrophobic, so it combines with proteins to travel through the blood.
Lipids are essential for long-term energy storage. Phospholipids and cholesterol act as part of the cell membrane.
Link to Real-Life Situations:
People eat lipids all the time in their food, and lipids are very filling. People get cholesterol from their liver and from foods they eat. Cholesterol helps their bodies create bile, hormones and vitamin D.
Lipids are made of carbon, oxygen, and hydrogen. They have no true monomers, but they do have subunits. They are mostly non-polar and cannot be dissolved in water.
Most lipids are fatty acids, which are made up of triglycerides, which are made up of one glycerol and three fatty acid tails. Combining these together makes polymers. Phospholipids, however, is made of one glycerol, two fatty acid tails, and one phosphate group head.
Phospholipids have a very unique structure: its head is hydrophilic and the tails are hydrophobic. One side of the phospholipids attracts water while the other side attracts other phospholipids. Ths is the structure of cell membranes.
Nucleic Acids
Polymer: DNA
Roles
Nucleic Acids store and move genetic information
DNA is made of many nucleotides that each contain a different nitrogenous base: Adenine, Thymine, Guanine, or Cytosine. The order than those bases are in in a long chain determines an individual's genetic information. RNA is structured almost identically, and it moves the gentic information from the DNA to the cytoplasm.
Without DNA or RNA, there would be nothing to store or move genetic information. Then, there would be no living things anywhere.
Link to Real-Life Situations:
All people, animals, and plants are at least a little different, and those differences are encoded in their DNA.
Structure and Properties
Nucleic Acids contain carbon, hydrogen, oxygen, nitrogen, and phosphorous. They are made up of monomers called nucleotides. They can be dissolved in water and are polar.
Nucleotides are made of three basic groups: the 5-carbon sugar, the nitrogenous base, and the phosphate group. Combining many nucleotides creates DNA or RNA, both of which are essential to life.