What is it...
Can develop into...
Combined with carboxylic acid to
create...
Combined with carbonyl group to
create...
Named...
The 3 types are...
Mainly used in...
Can be split between the following...
This is also known as an...
Can either be connected with...
Could either have...
Water used in reaction
Water created
Functional groups replaced
Bond to C lost
Bond to O lost
Bond to O created
Bond to C created
Can be either an...
Named by...
Which can be grouped by...
Help...
Example...
Are the...
Could either be...
Determine the...
Find any...
Then the...
Starts off with...
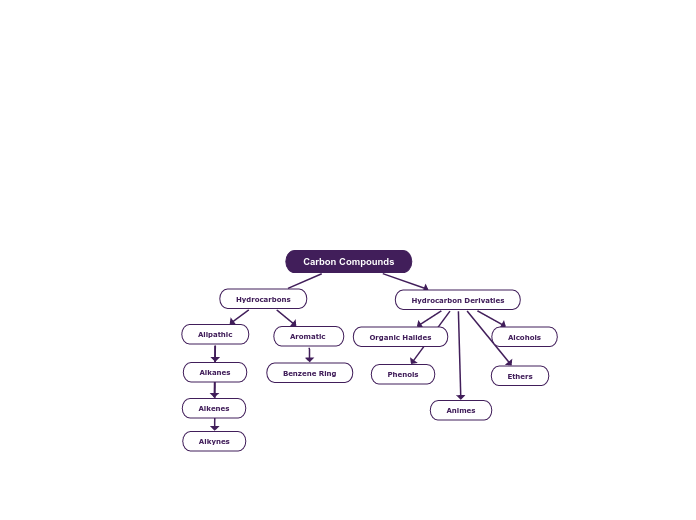
Cylic Hydrocarbons
Cycloalkenes
Cyclohexene
Cylcloalkanes
Cyclopentane
Root: -cyclo
Aromatic Hydrocarbons
Benzene/Phenyl
Methylbenzene
When carbon chain has more than 6 carbons the benzene is considered a side group and named phenyl group
Suffix: no suffix
Root: -benzene
Naming
4) Suffix
Remove -e and
add -ane
Indicates what type of hydrocarbon it is
3) Prefix
4th postion and 2
carbons in a branch
Branches
Number of carbons that aren't connected to the parent chain
Given postions and names of any branches
2) Root
8 carbon atoms
Denotes the number of C atoms in longest parent chain
1) Parent Chain
Longest carbon chain
Is the longest chain of carbons
Basic Alkene/ane/yne
Unsaturated
Due to double/triple
bond, less H bonds with
C atoms
Alkynes
Propyne
Suffix: -yne
Triple bond between carbons
Alkenes
Trans
An isomer in which
largest groups on C
atoms are attached on
the opposite sides
Trans-2-butene
Cis
An isomer in which
largest groups on C
atoms are attached on
the same side
Cis-2-butene
Propene
Suffix: -ene
Double bond between carbons
Saturated
More H bonds with
C atoms due to single
bond
Alkanes
Propane
Suffix: -ane
Each molecule differes from prevous by adding -CH2- (homologous series)
Single bonds between carbons
Functional Groups
Double Bonded Groups
Carbonyl Group
Ketones
Suffix: -one
Butanone
Amides
Suffix: -amide
Methanamide
Aldehydes
Suffix: -al
Methanal
Carboxylic Acids
Esters
Suffix: -oate
Propyl Ethanoate
Suffix: -oic acid
Propanioic Acid
Single Bonded Groups
Amines
Suffix: -amine
Propan-1-amine
Ethers
Adding -oxy to branches
2-ethoxypropane
Haloalkanes/Alkyl Halides
Named with prefixes:
(fluoro-,chloro-,bromo-,iodo-)
Trichloromethane
Hydroxyl Group
Alchohols
Tertiary
The carbon with the
hydroxyl is bonded to
three other carbon
C has no H bonds and 3 R
bonds
Secondary
The carbon with the
hydroxyl is bonded to
two other carbon
C has 1 H bond and 2 R
bonds
Primary
The carbon with the
hydroxyl is bonded to
one other carbon
C has 2 H bonds and 1 R
bond
Multiple -OHs
Diol, Triol, Tetra
Ethane-1,1-diol
Suffix: -ol
Ethanol
Has...
[
Organic Nomenclature
Reactions
Two or more products
Hydrolysis
When water splits a bond into two
Condensation
Two organic molecules join to form one organic molecule with water molecule
Elimination
Reverse of addition where an atom is removed to from a double bond
Substitution
When a functional groupe is replaced with another group
One product
Oxidization
When a reactant is oxidized an another is reduced
Reduction
Creates more bonds with H and fewer with O
Addition
When atoms are added to form a double or triple bond