da Anwar :D mancano 20 ore
3
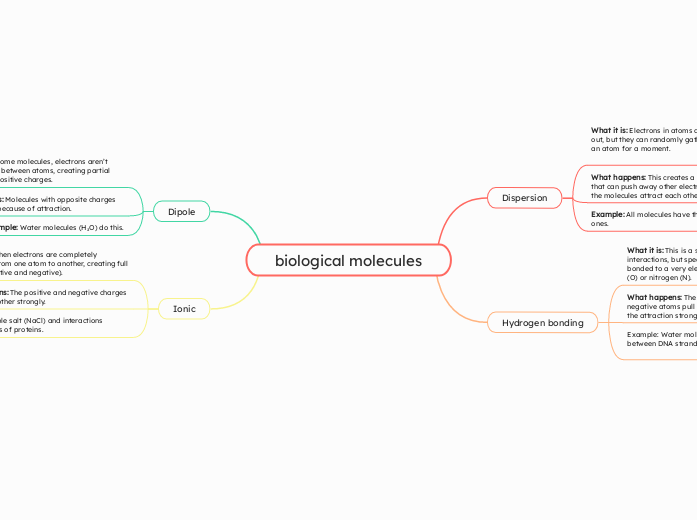
biological molecules
Within molecular interactions, different forces play a crucial role in the behavior and properties of substances. Dipoles occur when electrons are unequally shared between atoms, leading to molecules with partial positive and negative charges that attract each other.