da AA - 07TO 982485 Sir John A Macdonald Sr PS mancano 5 mesi
147
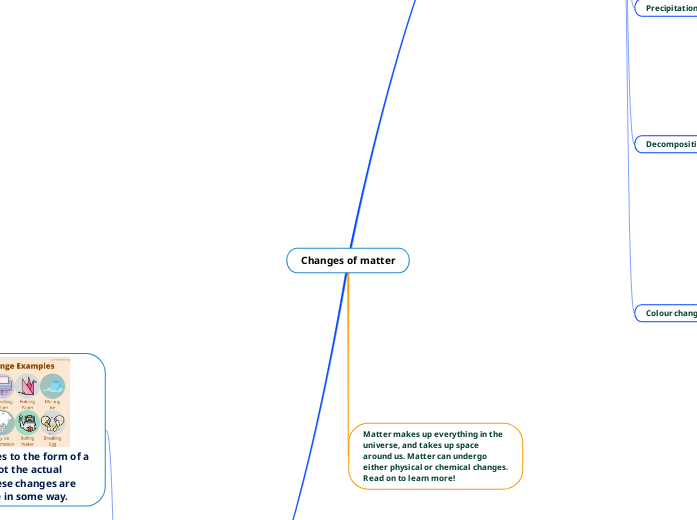
Changes of matter
The transformation of matter can be categorized into physical and chemical changes. Physical changes, such as dissolving, freezing, melting, boiling, and chopping, alter the form of a substance without changing its chemical composition and are typically reversible.