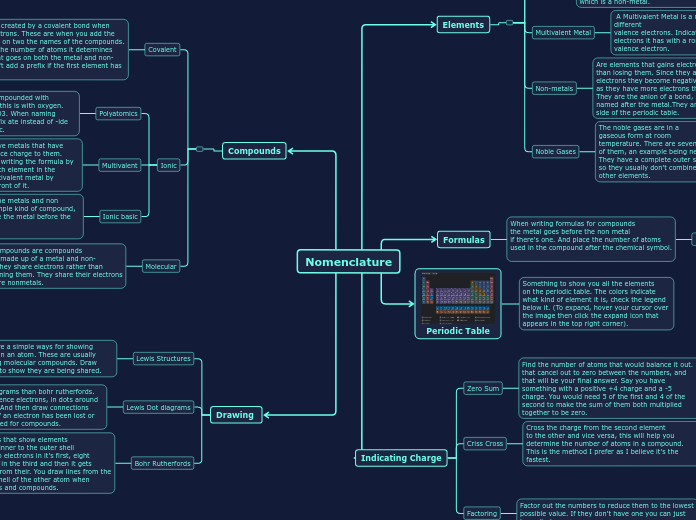
Nomenclature
Drawing
Bohr Rutherfords
These are diagrams that show elements
electrons from the inner to the outer shell
An electron has two electrons in it's first, eight
in the second eight in the third and then it gets
more complicated from their. You draw lines from the outer shell to the shell of the other atom when drawing it for bonds and compounds.
Lewis Dot diagrams
These are simpler diagrams than bohr rutherfords.
You only draw the valence electrons, in dots around the chemical symbol. And then draw connections with arrows to show if an electron has been lost or gained when being used for compounds.
Example
Lewis Structures
Lewis structures are a simple ways for showing
the valence shells in an atom. These are usually used when drawing molecular compounds. Draw lines to the atoms to show they are being shared.
Example: Carbon Dioxide
Indicating Charge
Factoring
Factor out the numbers to reduce them to the lowest
possible value. If they don't have one you can just leave it alone.
Example: Octane
Criss Cross
Cross the charge from the second element
to the other and vice versa, this will help you
determine the number of atoms in a compound.
This is the method I prefer as I believe it's the fastest.
Example: Sodium Phosphide
If it's a multivalent un cross the numbers
to get their charge they specify the multivalent
metal by indicating it's charged by a roman numeral.
Zero Sum
Find the number of atoms that would balance it out. that cancel out to zero between the numbers, and that will be your final answer. Say you have something with a positive +4 charge and a -5 charge. You would need 5 of the first and 4 of the second to make the sum of them both multiplied together to be zero.
Periodic Table
Something to show you all the elements
on the periodic table. The colors indicate
what kind of element it is, check the legend
below it. (To expand, hover your cursor over
the image then click the expand icon that
appears in the top right corner).
Formulas
When writing formulas for compounds
the metal goes before the non metal
if there's one. And place the number of atoms
used in the compound after the chemical symbol.
Ex. Octane= C8H18
Elements
Compounds