da Angelina Blakey mancano 5 anni
321
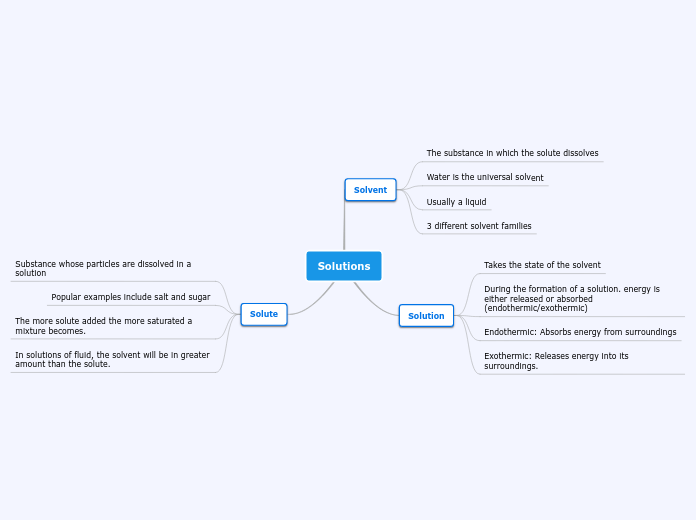
Solutions
When forming a solution, energy changes occur, either absorbing or releasing energy. This process can be classified as endothermic, where energy is absorbed from the surroundings, or exothermic, where energy is released into the surroundings.