da Jami Muse mancano 5 anni
239
Tree organigram
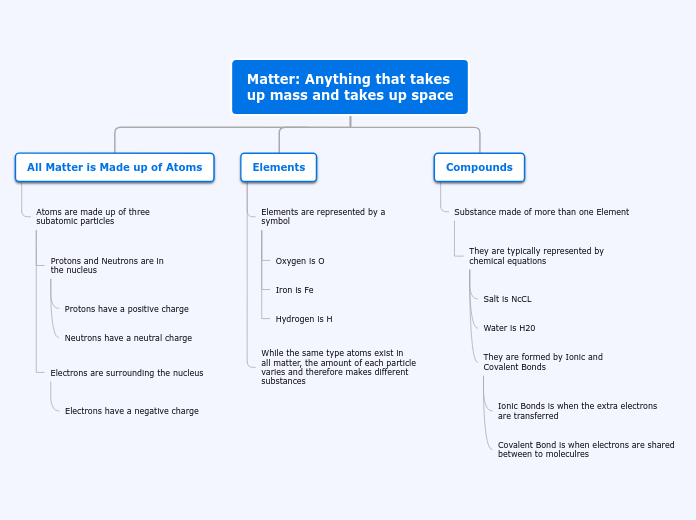
Matter is defined as anything that has mass and occupies space, and it is composed of atoms. Atoms, the fundamental building blocks of matter, consist of three subatomic particles: protons, neutrons, and electrons.